Volume 25, Number 3—March 2019
Synopsis
Donor-Derived Genotype 4 Hepatitis E Virus Infection, Hong Kong, China, 2018
Figure 4
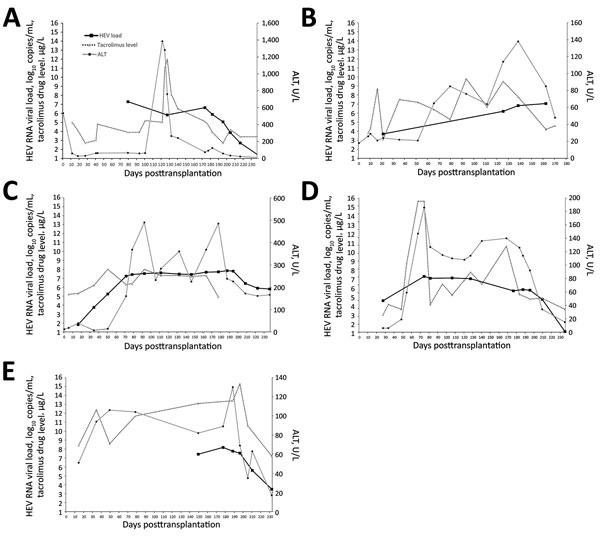
Figure 4. Kinetics of liver function test (ALT) results, tacrolimus levels, and plasma HEV RNA load with relation to ribavirin therapy. A) Case-patient 1; B) case-patient 2; C) case-patient 3; D) case-patient 4; E) case-patient 5. Date for case-patients 1, 3, 4, and 5 were updated up to week 8 of ribavirin treatment. Horizontal black bars indicate when patient began taking oral ribavirin. ALT, alanine aminotransferase; HEV, hepatitis E virus.
Page created: February 19, 2019
Page updated: February 19, 2019
Page reviewed: February 19, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.