Volume 25, Number 7—July 2019
Dispatch
Carbapenem and Cephalosporin Resistance among Enterobacteriaceae in Healthcare-Associated Infections, California, USA1
Abstract
We analyzed antimicrobial susceptibility test results reported in healthcare-associated infections by California hospitals during 2014–2017. Approximately 3.2% of Enterobacteriaceae reported in healthcare-associated infections were resistant to carbapenems and 26.9% were resistant to cephalosporins. The proportion of cephalosporin-resistant Escherichia coli increased 7% (risk ratio 1.07, 95% CI 1.04–1.11) per year during 2014–2017.
The Centers for Disease Control and Prevention (CDC) identified carbapenem-resistant Enterobacteriaceae (CRE) as an urgent public health threat and extended-spectrum β-lactamase (ESBL)–producing Enterobacteriaceae as a serious public health threat (1). Antimicrobial-resistant pathogens, such as CRE, can spread across regions when infected or colonized patients transfer between healthcare facilities without infection control measures in place to prevent transmission (2). Therefore, tracking regional changes in antimicrobial resistance (AMR) is essential to inform public health prevention and containment strategies.
Healthcare-associated infection (HAI) pathogen data reported to the National Healthcare Safety Network (NHSN) can be used to estimate the prevalence of AMR among hospitals within a region (3–5). Hospitals provide pathogen and antimicrobial susceptibility test results for <3 microorganisms when reporting central line–associated bloodstream infections (CLABSI), surgical site infections (SSI), and catheter-associated urinary tract infections (CAUTI) to NHSN (6). Data on molecular mechanisms of resistance are not collected for CLABSI, SSI, or CAUTI.
We applied CDC definitions to identify antimicrobial-resistant phenotypes among Enterobacteriaceae, including Escherichia coli, Klebsiella species, and Enterobacter species, reported in CLABSI, SSI, and CAUTI by general acute-care hospitals in California (3). We included multiple pathogens per HAI if reported. California hospitals report HAI data for <28 surgical procedures; we included pathogen data from any SSI reported. We excluded HAI data reported by other hospital types, such as critical access and long-term acute-care hospitals, due to limited HAI data reported by these hospitals.
According to CDC definitions, CRE were resistant to imipenem, meropenem, doripenem, or ertapenem. Extended-spectrum cephalosporin-resistant (ESCR) Enterobacteriaceae were resistant to ceftriaxone, ceftazidime, cefepime, or cefotaxime. We applied modified phenotype definitions from Magiorakos et al. to identify multidrug-resistant (MDR), extensively drug-resistant (XDR), and pandrug-resistant (PDR) Enterobacteriaceae (7). Susceptibility data for 2 antimicrobial drugs (ceftaroline and fosfomycin) included in these definitions were not available in our NHSN data. Resistance was defined by an isolate’s nonsusceptibility to >1 agent (e.g., imipenem) within a category of antimicrobial drugs (e.g., carbapenems) and the total number of antimicrobial categories (<15) for which the isolate was nonsusceptible. MDR Enterobacteriaceae were nonsusceptible to >3 antimicrobial categories; XDR Enterobacteriaceae were nonsusceptible to all but 1 or 2 antimicrobial categories, and PDR Enterobacteriaceae were nonsusceptible to all antimicrobial categories. We also assessed the phenotype difficult-to-treat (DTR) proposed by Kadri et al. (8). DTR included an intermediate or resistant result to all reported agents within carbapenem, cephalosporin, and fluoroquinolone categories, as well as piperacillin-tazobactam and aztreonam when results were available.
We used log binomial regression models to estimate statewide, year-to-year change in the proportion of antimicrobial-resistant Enterobacteriaceae during 2014–2017. To understand regional differences in CRE and ESCR Enterobacteriaceae, we performed a subgroup analysis in which we aggregated HAI data in 2-year increments and measured percentage resistance by county when susceptibility test results for >30 Enterobacteriaceae were available. CDC has explored risk adjustment for regional-level comparisons using NHSN data and determined unadjusted measures are satisfactory until additional covariates are adopted in NHSN (9).
We completed data analyses in SAS version 9.4 (SAS, http://www.sas.com) and spatial analyses in ArcMap version 10.4 (Environmental Systems Research Institute, Inc., https://www.esri.com). This public health surveillance analysis met criteria for nonresearch activity and did not require an exemption determination from the California Committee for the Protection of Human Subjects.
During 2014–2017, 305 (91%) of 335 California hospitals reported >1 Enterobacteriaceae in HAI with cephalosporin susceptibility test results; 296 (88%) hospitals reported >1 Enterobacteriaceae with carbapenem susceptibility test results. The median number of Enterobacteriaceae reported with cephalosporin susceptibility test results by hospitals per year was 8 (interquartile range 16–3), and 6 (interquartile range 14–3) for Enterobacteriaceae with carbapenem susceptibility test results.
Approximately 3.2% of Enterobacteriaceae reported in HAI during 2014–2017 were resistant to carbapenems and 26.9% of Enterobacteriaceae reported in HAI were cephalosporin resistant. We observed increases in the proportions of Enterobacteriaceae that were ESCR and MDR during 2014–2017; these changes were driven by E. coli (Table 1). We observed a 7% (risk ratio [RR] 1.07; 95% CI 1.04–1.11) annual increase in the proportion of E. coli resistant to cephalosporins and a 4% (RR 1.04; 95% CI 1.02–1.06) annual increase in the proportion of E. coli with an MDR phenotype during 2014–2017 (Table 1). The proportion of E. coli exhibiting carbapenem resistance also increased 24% (RR 1.24; 95% CI 1.00–1.56) per year during 2014–2017.
We observed decreasing trends in carbapenem resistance (RR 0.90; 95% CI 0.80–1.01) and in the DTR phenotype (RR 0.88; 95% CI 0.77–1.00) among Klebsiella species reported in HAI. Among Enterobacteriaceae assessed for the DTR phenotype, Klebsiella species accounted for 86% (n = 193) of DTR isolates and comprised 23% of the overall total of Enterobacteriaceae analyzed among HAI. In addition, 1 XDR Klebsiella pneumoniae was reported in HAI during 2014–2017 and no PDR Enterobacteriaceae were reported.
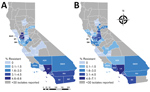
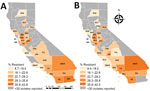
Percentages of CRE and ESCR phenotypes varied by county and reporting years (Table 2; Figures 1, 2). Carbapenem and cephalosporin resistance was higher in California regions more densely populated with hospitals and residents, such as the greater Los Angeles region and San Francisco Bay area. Counties with hospitals reporting <30 Enterobacteriaceae may still have antimicrobial-resistant HAI or receive patients from healthcare facilities where antimicrobial resistance is endemic.
Several factors limit the interpretation of our results. Only 4 years of data were available for measuring AMR trends. Selective reporting of susceptibility test results may have restricted sample sizes and increased the potential for sampling bias to affect our results. Furthermore, there may be differences in how California hospitals and laboratories interpret MIC breakpoints or changes in how breakpoints are applied over time. Data on molecular mechanisms of resistance are not collected in CLABSI, SSI, or CAUTI, which limits our understanding of how transmissible elements, including ESBL and carbapenemases, may contribute to the trends we observed.
Increases in carbapenem, cephalosporin, and MDR E. coli reported in HAI by California hospitals are concerning, given that E. coli are common causes of both hospital and community-associated infections. ESBL-producing E. coli have been reported in community-associated urinary tract infections among patients in California, with estimates of resistance among E. coli from 5% up to 17% in complicated pyelonephritis (10,11). MDR and DTR Enterobacteriaceae further limit treatment options and present management challenges, particularly in outpatient settings when there are no oral antimicrobial treatment options.
AMR prevention and containment strategies may depend on the local prevalence. For example, prompt detection and rapid, aggressive containment responses to individual AMR cases can be effective in low-prevalence regions. Admission screening and empiric use of transmission-based precautions for patients at high risk for AMR might be more feasible in higher-prevalence regions.
Healthcare facilities can prevent HAI and the spread of AMR by implementing best practices in infection control and antimicrobial stewardship. State and local health departments can coordinate prevention efforts across the healthcare continuum, investigate and control outbreaks in healthcare facilities, and set expectations for healthcare facilities to communicate patients’ AMR infection and colonization status during all patient transfers. Decreasing trends in carbapenem resistance and in the DTR phenotype among Klebsiella species, often the focus of AMR containment efforts, indicate the potential effectiveness of such prevention strategies (5). Nonetheless, increases and regional variation in carbapenem-resistant and ESCR E. coli highlight the urgent need for ongoing, local infection prevention and antimicrobial stewardship efforts.
Mr. Rizzo is an epidemiologist with the Healthcare-Associated Infections Program at the California Department of Public Health. His work focuses on surveillance of antimicrobial-resistant healthcare-associated infections and evaluation of prevention programs.
Acknowledgment
Aspects of this work were supported by Centers for Disease Control and Prevention Epidemiology and Laboratory Capacity grant funds.
References
- Centers for Disease Control and Prevention. Antibiotic/antimicrobial resistance. Biggest threats and data. 2018 [cited 2019 Feb 19]. https://www.cdc.gov/drugresistance/biggest_threats.html
- Huang SS, Avery TR, Song Y, Elkins KR, Nguyen CC, Nutter SK, et al. Quantifying interhospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol. 2010;31:1160–9. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Patient safety atlas. 2018 [cited 2018 Jul 13]. https://www.cdc.gov/hai/surveillance/ar-patient-safety-atlas.html
- Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, Kallen AJ, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37:1288–301. DOIPubMedGoogle Scholar
- Woodworth KR, Walters MS, Weiner LM, Edwards J, Brown AC, Huang JY, et al. Vital signs: containment of novel multidrug-resistant organisms and resistance mechanisms—United States, 2006–2017. MMWR Morb Mortal Wkly Rep. 2018;67:396–401. DOIPubMedGoogle Scholar
- National Healthcare Safety Network, Centers for Disease Control and Prevention. Surveillance reporting for enrolled facilities, 2015 [cited 2018 Jul 10]. https://www.cdc.gov/nhsn/enrolled-facilities/index.html
- Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–81. DOIPubMedGoogle Scholar
- Kadri SS, Adjemian J, Lai YL, Spaulding AB, Ricotta E, Prevots DR, et al.; National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH–ARORI). Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin Infect Dis. 2018;67:1803–14. DOIPubMedGoogle Scholar
- Soe MM, Edwards JR, Sievert DM, Ricks PM, Magill SS, Fridkin SK. Evaluating state-specific antibiotic resistance measures derived from central line-associated bloodstream infections, national healthcare safety network, 2011. Infect Control Hosp Epidemiol. 2015;36:54–64. DOIPubMedGoogle Scholar
- Talan DA, Takhar SS, Krishnadasan A, Abrahamian FM, Mower WR, Moran GJ. EMERGEncy ID Net Study Group. Fluoroquinolone-resistant and extended-spectrum β-lactamase–producing Escherichia coli infections in patients with pyelonephritis, United States. Emerg Infect Dis. 2016;•••:22.
- Frazee BW, Trivedi T, Montgomery M, Petrovic D-F, Yamaji R, Riley L. Emergency department urinary tract infections caused by extended-spectrum β-lactamase–producing Enterobacteriaceae: many patients have no identifiable risk factor and discordant empiric therapy is common. Ann Emerg Med. 2018;72:449–56. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 12, 2019
1Preliminary data from this analysis were presented in a poster at IDWeek 2018 in San Francisco, California, USA, October 5, 2018.
Table of Contents – Volume 25, Number 7—July 2019
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Kyle Rizzo, California Department of Public Health, Healthcare-Associated Infections Program, 850 Marina Bay Pkwy, Building E, 1st Floor, Richmond, CA 94804, USA
Top