Volume 25, Number 9—September 2019
Dispatch
Use of Human Intestinal Enteroids to Detect Human Norovirus Infectivity
Figure 1
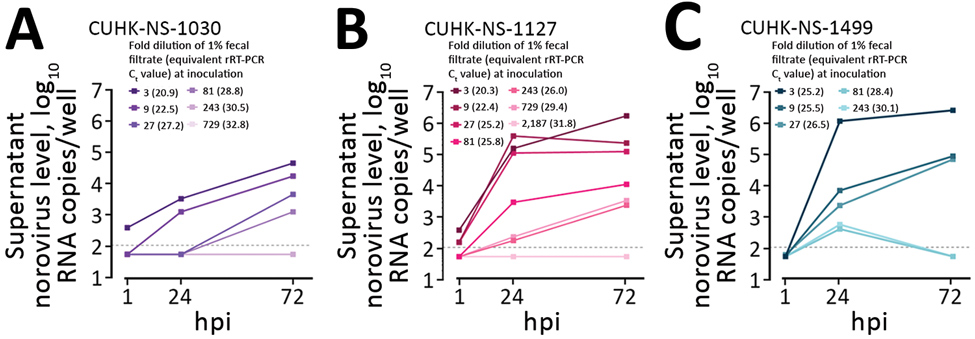
Figure 1. Performance of a human intestinal enteroid (HIE) line and an antigen-based enzyme immunoassay (EIA) to detect human norovirus in clinical fecal samples from 3 patients in Hong Kong. Replication kinetics of 3 human pandemic norovirus genogroup II genotype 4 (GII.Pe-GII.4 Sydney) strains (CUHK-NS-1030, from a 1-year-old boy; CUHK-NS-1127, from a 79-year-old man; CUHK-NS-1499, from a 46-year-old man) were tested in a monolayer culture of the adult stem cell–derived HIE line J2. We used 3-fold serial dilutions of norovirus-containing fecal filtrates to challenge J2 seeded on 96-well cell culture plates. Norovirus RNA levels in the culture supernatant at the indicated times were measured by rRT-PCR by using a 10-fold serially diluted standard of in vitro–transcribed norovirus RNA of genotype GII.Pe-GII.4 Sydney. Horizontal dotted lines denote the lower limit of detection of the rRT-PCR assay (13.8 RNA copies/reaction or 110 RNA copies/well) as determined by probit analysis. Samples with undetectable norovirus RNA were arbitrarily assigned a value equal to half of the lower limit of detection for calculation purpose. To convert the unit from RNA copies/well to RNA copies/mL, multiply the value by a factor of 10. hpi, hours postinfection; rRT-PCR, real-time reverse transcription PCR.