Volume 26, Number 2—February 2020
Research
Unique Clindamycin-Resistant Clostridioides difficile Strain Related to Fluoroquinolone-Resistant Epidemic BI/RT027 Strain
Figure 3
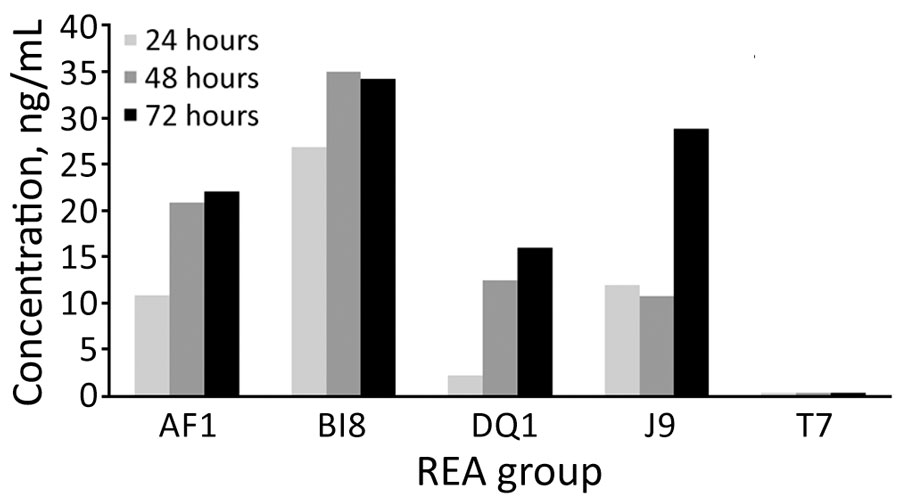
Figure 3. Quantitative in vitro total toxin production in study of C. difficile at 2 US Veteran Affairs long-term care facilities and their affiliated acute care facilities. Results at 24, 48, and 72 hours of incubation are shown for REA strains AF (ribotype 244), BI (ribotype 027), DQ (ribotype 591), J (ribotype 001), and T (a nontoxigenic Clostridioides difficile strain). REA, restriction endonuclease analysis.
Page created: January 17, 2020
Page updated: January 17, 2020
Page reviewed: January 17, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.