Volume 26, Number 8—August 2020
Research
Linezolid-Associated Neurologic Adverse Events in Patients with Multidrug-Resistant Tuberculosis, France
Figure 1
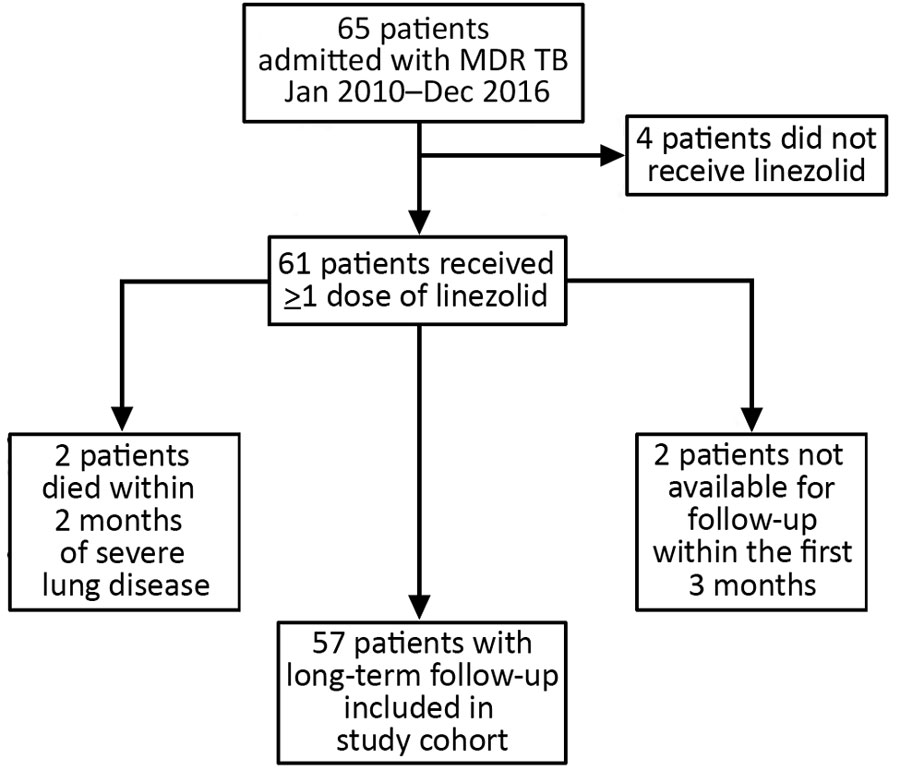
Figure 1. Outcomes for 65 patients with multidrug-resistant tuberculosis (MDR TB) admitted to Pitié-Salpêtrière Hospital, Paris, France, and included in study of linezolid-associated neurologic adverse events.
Page created: June 12, 2020
Page updated: July 17, 2020
Page reviewed: July 17, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.