Volume 27, Number 1—January 2021
Synopsis
Impact of Human Papillomavirus Vaccination, Rwanda and Bhutan
Figure 2
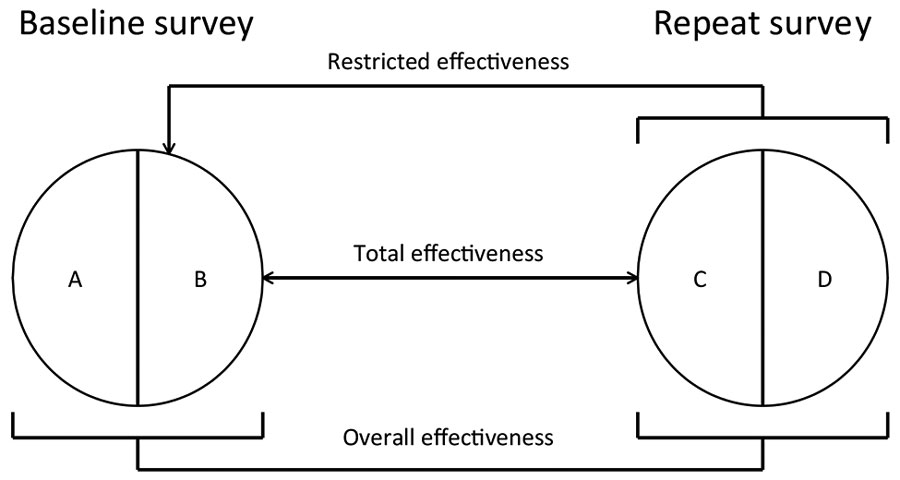
Figure 2. Analytical framework used to assess the impact of human papillomavirus (HPV) vaccination in Rwanda and Bhutan. A) Vaccinated participants in the baseline survey. B) Unvaccinated participants in the baseline survey. C) Vaccinated participants in the repeat survey. D) Unvaccinated participants in the repeat survey. Vaccine effectiveness (VE) was calculated as VE = (1 – PR)%, where PR is a prevalence ratio (PR). Each type of VE is defined according to specific criteria for selecting comparison groups on the basis of reported vaccination status. Overall effectiveness estimates, providing a measure of HPV prevalence reduction over time attributable to vaccination irrespective of the reported vaccination status of each person, were obtained by comparing the type-specific HPV prevalence in all women, unvaccinated and vaccinated, recruited in the baseline and repeat surveys. PR (C and D) / PR (A and B) = overall PR. Restricted effectiveness estimates, providing an approximate estimate of the impact of HPV vaccination versus an entirely unvaccinated population, were obtained by comparing the type-specific HPV prevalence in unvaccinated women in the baseline and all women in repeat surveys. PR (C and D) / PR (B) = restricted PR. Total effectiveness estimates, providing a vaccine efficacy estimate (similar to measures from clinical trials) from real-life settings, were obtained by comparing the type-specific HPV prevalence in unvaccinated women in the baseline and vaccinated women in repeat surveys. PR (C) / PR (B) = total PR, where PR (•) is the type-specific HPV prevalence in each participant group.