Volume 27, Number 1—January 2021
Synopsis
Impact of Human Papillomavirus Vaccination, Rwanda and Bhutan
Figure 3
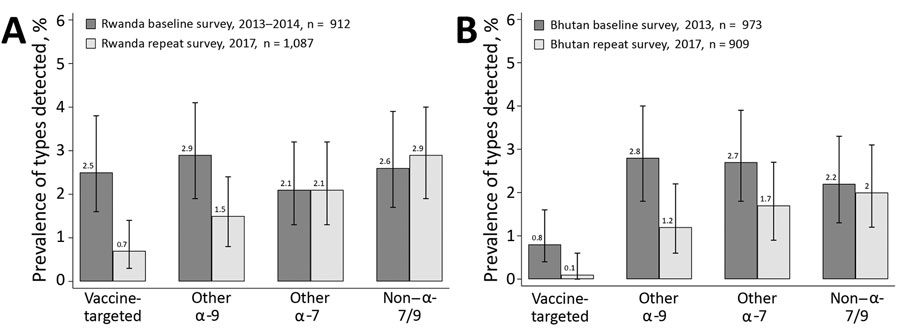
Figure 3. Overall crude human papillomavirus prevalence by general primer GP5+/6+-mediated PCR in baseline and repeat surveys in Rwanda (A) and Bhutan (B), with corresponding 95% CIs. Vaccine-targeted types (HPV-6, -11, -16, -18); other α-9 types (HPV-31, -33, -35, -52, -58); other α-7 types (HPV-39, -45, -59, -68); non–α 7/9 types (HPV-26, -51, -53, -56, -66, -70, -73, -82).
Page created: November 03, 2020
Page updated: December 21, 2020
Page reviewed: December 21, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.