Volume 27, Number 1—January 2021
Letter
Large-Scale Isolation Facilities and Potential for Secondary Infectious Disease Outbreak
Cite This Article
Citation for Media
To the Editor: Singapore has instituted large-scale isolation facilities similar to those detailed by Choi et al. (1) for patients with mild coronavirus disease. We highlight the risk for transmission of secondary infectious diseases by sharing our experience with a varicella outbreak.
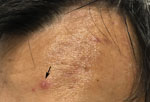
Three patients, all migrant workers housed in the same isolation hall, were seen for vesicular eruptions, later laboratory confirmed as varicella, within the span of 9 days. The first patient’s symptoms were truncal erythematous-based vesicles and erosions after a prodrome of fever and headache. He was promptly transferred for further hospital isolation. As part of a ring vaccination strategy, we offered 200 close contacts postexposure vaccination. However, 2 other patients, not close contacts of the first, had similar eruptions; for the second patient, 7 days later with a rash duration of 2 days, and for the third, 8 days after, with a rash duration of 6 days (Figure). After these additional cases, vaccination was offered to all remaining patients in the isolation facility.
All 3 patients probably contracted varicella from unidentified persons with varicella or zoster infection, given that illness onset fell short of the usual 10–21-day incubation period (2). Although varicella seroprevalence among adults in Singapore is high (88%), data on seroprevalence among migrant workers remain limited (3).
Although isolation facilities obviate the capacity constraints of hospital isolation, our experience highlights the potential for secondary outbreaks, which are disruptive and costly to investigate and control. To mitigate this risk, preentry screening inquiring about previous chickenpox infection or vaccination should be considered. Serologic screening is ideal but challenging to implement. Among patients, social distancing and face coverings should be enforced. We also recommend active surveillance for vesicular rash and fever, prompt isolation of patients with suspected cases, and vaccination of identified close contacts without previous infection, vaccination, or contraindications to vaccination, as well as temporarily halting patient flow while these measures are implemented.
Acknowledgment
The initial patient described in this article has given his consent for his image and other clinical information to be reported. The patient understands that his name and initials will not be published, and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
References
- Choi WS, Kim HS, Kim B, Nam S, Sohn JW. Community treatment centers for isolation of asymptomatic and mildly symptomatic patients with coronavirus disease, South Korea. Emerg Infect Dis. 2020;26:2338–45. DOIPubMedGoogle Scholar
- Czumbel I, Quinten C, Lopalco P, Semenza JC; ECDC expert panel working group. Management and control of communicable diseases in schools and other child care settings: systematic review on the incubation period and period of infectiousness. BMC Infect Dis. 2018;18:199. DOIPubMedGoogle Scholar
- Fatha N, Ang LW, Goh KT. Changing seroprevalence of varicella zoster virus infection in a tropical city state, Singapore. Int J Infect Dis. 2014;22:73–7. DOIPubMedGoogle Scholar
Figure
Cite This Article1These authors contributed equally to this article.
Related Links
Table of Contents – Volume 27, Number 1—January 2021
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Shi Yu Derek Lim, National Skin Centre, Singapore, 1 Mandalay Rd 308205, Singapore
Top