Volume 27, Number 11—November 2021
Research
Hepatitis A Virus Incidence Rates and Biomarker Dynamics for Plasma Donors, United States
Figure 3
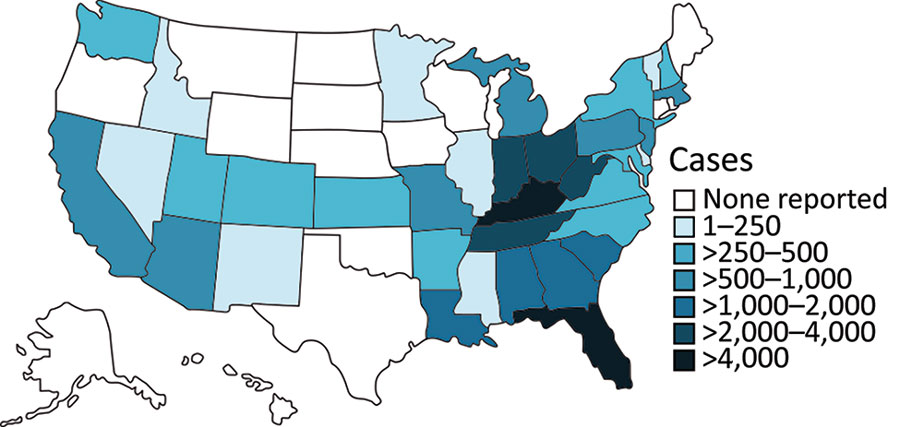
Figure 3. Geographic distribution of CDC-reported HAV outbreak cases, United States, as of February 2021, showing state-reported HAV outbreak cases as listed on the CDC website for the current outbreak since August 2016 (7). Outbreak-associated status is determined at state level in accordance with the respective outbreak case definition for each state. Therefore, HAV cases not classified as outbreak-associated are probably not captured in CDC data. CDC, Centers for Disease Control and Prevention; HAV, hepatitis A virus.
References
- Hollinger FB, Martin A. Hepatitis A virus. In: Knipe DM, Howley PM, editors. Fields virology, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. p.550–81.
- Martin A, Lemon SM, Hepatitis A. Hepatitis A virus: from discovery to vaccines. Hepatology. 2006;43(Suppl 1):S164–72. DOIPubMedGoogle Scholar
- Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, et al. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–71. DOIPubMedGoogle Scholar
- Asher LV, Binn LN, Mensing TL, Marchwicki RH, Vassell RA, Young GD. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus). J Med Virol. 1995;47:260–8. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Viral hepatitis surveillance—United States, 2015 [cited 2020 Sep 5]. https://www.cdc.gov/hepatitis/statistics/SurveillanceRpts.htm
- Centers for Disease Control and Prevention. Viral hepatitis Surveillance—United States, 2018 [cited 2020 Sep 5]. https://www.cdc.gov/hepatitis/statistics/SurveillanceRpts.htm
- Centers for Disease Control and Prevention. Widespread outbreaks of hepatitis A across the United States [cited 2021 Mar 4]. https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm
- Yin S, Barker L, Ly KN, Kilmer G, Foster MA, Drobeniuc J, et al. Susceptibility to hepatitis A virus infection in the United States, 2007–2016. Clin Infect Dis. 2020;71:e571–9. DOIPubMedGoogle Scholar
- World Health Organization. The immunological basis for immunization series: module 18: hepatitis A. 2019, 1–68 [cited 2021 Aug 2]. https://apps.who.int/iris/bitstream/handle/10665/326501/97892516327-eng.pdf
- Foster M, Ramachandran S, Myatt K, Donovan D, Bohm S, Fiedler J, et al. Hepatitis A virus outbreaks associated with drug use and homelessness—California, Kentucky, Michigan, and Utah, 2017. MMWR Morb Mortal Wkly Rep. 2018;67:1208–10. DOIPubMedGoogle Scholar
- European Pharmacopeia. Human plasma for fractionation (monograph 853) [cited 2021 Aug 2]. https://pharmacopoeia.ru/en/fs-3-3-2-0001-15-plazma-cheloveka-dlya-fraktsionirovaniya
- Plasma Protein Therapeutics Association. International quality plasma program standards on donor health and donor management [cited 2021 Aug 2]. https://www.pptaglobal.org/safety-quality/standards/iqpp
- Gowland P, Fontana S, Niederhauser C, Taleghani BM. Molecular and serologic tracing of a transfusion-transmitted hepatitis A virus. Transfusion. 2004;44:1555–61. DOIPubMedGoogle Scholar
- Marosevic D, Belting A, Schönberger K, Carl A, Wenzel JJ, Brey R. Hepatitis A outbreak in the general population due to a MSM-associated HAV genotype linked to a food handler, November 2017 – February 2018, Germany. Food Environ Virol. 2019;11:149–56. DOIPubMedGoogle Scholar
- Foster MA, Hofmeister MG, Kupronis BA, Lin Y, Xia GL, Yin S, et al. Increase in hepatitis A virus infections—United States, 2013–2018. MMWR Morb Mortal Wkly Rep. 2019;68:413–5. DOIPubMedGoogle Scholar
- Gallian P, Barlet V, Mouna L, Gross S, Lecam S, Ricard C, et al. Hepatitis A: an epidemiological survey in blood donors, France 2015 to 2017. Euro Surveill. 2018;23:
1800237 . DOIPubMedGoogle Scholar - Weimer T, Streichert S, Watson C, Gröner A. Hepatitis A virus prevalence in plasma donations. J Med Virol. 2002;67:469–71. DOIPubMedGoogle Scholar
- Bower WA, Nainan OV, Han X, Margolis HS. Duration of viremia in hepatitis A virus infection. J Infect Dis. 2000;182:12–7. DOIPubMedGoogle Scholar
- Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999;59:2223–30.PubMedGoogle Scholar
- Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171(Suppl 1):S15–8. DOIPubMedGoogle Scholar
- European Medicines Agency. Guideline on plasma-derived medicinal products (EMA/CHMP/BWP/706271/2010). Effective date: February 1, 2012 [cited 2021 Aug 2]. https://pdf4pro.com/view/guideline-on-plasma-derived-medicinal-products-2324eb.html
- Plasma Protein Therapeutics Association. QSEAL NAT Testing Standard (version 2.0). Effective date: June 13, 2013 [cited 2021 Aug 2]. https://www.pptaglobal.org/safety-quality/standards/qseal
- Friesema IH, Sonder GJ, Petrignani MW, Meiberg AE, van Rijckevorsel GG, Ruijs WL, et al. Spillover of a hepatitis A outbreak among men who have sex with men (MSM) to the general population, the Netherlands, 2017. Euro Surveill. 2018;23:
1800265 . DOIPubMedGoogle Scholar - Cohen JI, Ticehurst JR, Feinstone SM, Rosenblum B, Purcell RH. Hepatitis A virus cDNA and its RNA transcripts are infectious in cell culture. J Virol. 1987;61:3035–9. DOIPubMedGoogle Scholar
- Costafreda MI, Abbasi A, Lu H, Kaplan G. Exosome mimicry by a HAVCR1-NPC1 pathway of endosomal fusion mediates hepatitis A virus infection. Nat Microbiol. 2020;5:1096–106. DOIPubMedGoogle Scholar
- Ott JJ, Wiersma ST. Single-dose administration of inactivated hepatitis A vaccination in the context of hepatitis A vaccine recommendations. Int J Infect Dis. 2013;17:e939–44. DOIPubMedGoogle Scholar
- Garner-Spitzer E, Kundi M, Rendi-Wagner P, Winkler B, Wiedermann G, Holzmann H, et al. Correlation between humoral and cellular immune responses and the expression of the hepatitis A receptor HAVcr-1 on T cells after hepatitis A re-vaccination in high and low-responder vaccinees. Vaccine. 2009;27:197–204. DOIPubMedGoogle Scholar
- Yoshikawa A, Gotanda Y, Itabashi M, Minegishi K, Kanemitsu K, Nishioka K. Hepatitis B NAT virus-positive blood donors in the early and late stages of HBV infection: analyses of the window period and kinetics of HBV DNA. Vox Sang. 2005;88:77–86. DOIPubMedGoogle Scholar
- Ribeiro RM, Qin L, Chavez LL, Li D, Self SG, Perelson AS. Estimation of the initial viral growth rate and basic reproductive number during acute HIV-1 infection. J Virol. 2010;84:6096–102. DOIPubMedGoogle Scholar
- Busch MP, Giachetti C, Gallarda J, Mimms LT, Peddada L, Charles H, et al. Dynamics of HCV viremia during early HCV infection: Implications for minipool vs. individual donation nucleic acid amplification testing. Transfusion. 2000;40:S25.
- Weusten JJ, van Drimmelen HA, Lelie PN. Mathematic modeling of the risk of HBV, HCV, and HIV transmission by window-phase donations not detected by NAT. Transfusion. 2002;42:537–48. DOIPubMedGoogle Scholar
- Qu L, Lemon SM. Hepatitis A and hepatitis C viruses: divergent infection outcomes marked by similarities in induction and evasion of interferon responses. Semin Liver Dis. 2010;30:319–32. DOIPubMedGoogle Scholar
Page created: August 02, 2021
Page updated: October 19, 2021
Page reviewed: October 19, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.