Volume 27, Number 4—April 2021
Research Letter
Rapid Spread and Control of Multidrug-Resistant Gram-Negative Bacteria in COVID-19 Patient Care Units
Cite This Article
Citation for Media
Abstract
We describe rapid spread of multidrug-resistant gram-negative bacteria among patients in dedicated coronavirus disease care units in a hospital in Maryland, USA, during May–June 2020. Critical illness, high antibiotic use, double occupancy of single rooms, and modified infection prevention practices were key contributing factors. Surveillance culturing aided in outbreak recognition and control.
Bacterial colonization and secondary infection have been described in patients hospitalized with coronavirus disease (COVID-19) (1,2). We report a single-center experience with spread of multidrug-resistant (MDR) gram-negative bacteria (GNB) in COVID-19 patients in Maryland, USA, during May–June 2020. This investigation was determined to be non–human subjects research by the University of Maryland’s Institutional Review Board.
At University of Maryland Medical Center (Baltimore, MD, USA), an 800-bed tertiary-care hospital, since early April 2020, critically ill COVID-19 patients had been housed in 3 dedicated units (3), which included 2 intensive care units (ICUs) (units A and B, unit A providing extracorporeal membrane oxygenation support) and 1 intermediate-care unit (unit C). Units were designed as closed, negative-pressure areas where staff remained in the same personal protective equipment while providing care to multiple patients. To accommodate the COVID-19 surge, single-patient ICU rooms in units A and B frequently housed 2 patients. Unit C rooms remained single-occupancy and received patients for step-down care from units A and B. Hospital policy required staff to change gloves and perform hand hygiene (or glove hygiene if wearing 2 layers of gloves) between patients and to wear 2 layers of gowns for patients with resistant organisms and remove the outer gown before moving to the next patient. A team nursing model was used, in which multiple nurses shared responsibilities for each patient during a shift.
For routine surveillance, the hospital defined MDR GNB as Enterobacterales, Acinetobacter baumannii, or Pseudomonas aeruginosa nonsusceptible to >2 of piperacillin/tazobactam, cefepime, and a carbapenem. Before COVID-19, we performed admission and weekly surveillance for MDR Enterobacterales and A. baumannii using perirectal swab specimens on medical and surgical ICU patients and monitored hospitalwide MDR GNB incidence by using the first positive clinical or surveillance culture >48 hours postadmission.
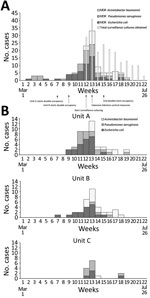
In mid-May 2020, a cluster of 4 patients with MDR Escherichia coli was identified on unit A. Hospitalwide data showed increase in MDR GNB incidence from baseline (Figure, panel A) (weeks 9–11), driven by E. coli cases on units A and B (Figure, panel B). Further review also revealed several patients with cefepime-resistant E. coli (not meeting institutional MDR criteria), MDR P. aeruginosa, and MDR A. baumannii. Surveillance screens (perirectal swab specimens on all and sputum on ventilated patients) in the 3 units in week 12 identified 18/29 (62%) additional patients with resistant GNB (MDR GNB, cefepime-resistant E. coli, or both). Public health authorities were notified and observations of practice and discussions with leadership were conducted. Twice-weekly surveillance culturing among patients still negative for resistant GNB was instituted (Figure).
During April 16–July 15, a total of 71 unique patients had positive clinical or surveillance cultures for resistant GNB, including 44 E.coli (33 MDR and 11 cefepime-resistant), 27 MDR P. aeruginosa, and 27 MDR A. baumannii (Appendix Table 1). Twenty-four patients (34%) were co-colonized with >1 resistant GNB. Of the 71 patients, 69 (97%) had received antibiotics before first positive resistant GNB culture, 30 (42%) required extracorporeal membrane oxygenation support, 27 (38%) required renal replacement therapy, 52 (73%) received corticosteroids, 25 (35%) received remdesivir, and 14 (20%) received tocilizumab. Twenty-three (32%) patients ultimately died.
Relatedness of early E. coli isolates was assessed by pulsed-field gel electrophoresis (PFGE) (n = 13, weeks 7–11) and genetic β-lactamase determination by Verigene gram-negative blood culture nucleic acid test (Luminex Corporation, https://www.luminexcorp.com) (n = 38, weeks 7–14) (4; Appendix). PFGE revealed 3 groups. Groups 1 and 2 (n = 7) were considered related and were negative for β-lactamases; these and 8/10 additional β-lactamase-negative isolates were from unit B. Group 3 (n = 6) isolates did not produce bands but were positive for CTX-M; these and 14/15 additional CTX-M positive isolates (including 10/11 phenotypically cefepime-resistant but not MDR) were from unit A and considered related, suggesting rapid patient-to-patient transmission (Appendix Table 1). MDR P. aeruginosa transmission occurred predominantly in unit A, whereas MDR A. baumannii was largely in unit B. Resistant GNB were likely introduced into unit C from both units A and B (Figure, panel B).
Key infection control findings (5) included tight physical spaces and close proximity of patients in double occupancy (6), multiple staff in contact with each patient in the team nursing model, and low compliance with hand and glove hygiene and gown changes between patients. To limit staff exposure to COVID-19 patients, the unit had less support from ancillary services; instead, daily room and equipment cleaning and stocking of medications and supplies were performed by unit-based clinical staff.
Outbreak control interventions included discontinuation of double occupancy, frequent infection prevention rounds to promote hand hygiene and glove and gown changes between patients, increased environmental services support, and attention to disinfection of reusable equipment and high-touch surfaces (Appendix Table 2) (7). Surveillance culturing showed a decrease in positive cultures over time (Figure).
Prolonged critical illness, high antibiotic and corticosteroid use, double occupancy, the team nursing model, and modified infection prevention practice were considered contributors to transmission, underscoring the importance of vigilance to MDR organisms in this setting (5,7–10). Surveillance culturing aided with recognizing the extent of spread and informed early intervention.
Dr. Patel is a second-year infectious diseases fellow at the University of Maryland Medical Center. She is interested in infection prevention and hospital epidemiology and has worked on projects involving hospital- acquired Clostridium difficile infections as well as hospital-onset bloodstream infections.
Acknowledgment
We would like to thank Richard Brooks and Heather Saunders for guidance on outbreak management, and Gwen Robinson for assistance with creating the figure.
References
- Rawson TM, Moore LSP, Castro-Sanchez E, Charani E, Davies F, Satta G, et al. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75:1681–4. DOIPubMedGoogle Scholar
- Nori P, Cowman K, Chen V, Bartash R, Szymczak W, Madaline T, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42:84–8. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). 2020 [cited 2020 Aug 20]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html
- Hill JT, Tran K-DT, Barton KL, Labreche MJ, Sharp SE. Evaluation of the nanosphere Verigene BC-GN assay for direct identification of gram-negative bacilli and antibiotic resistance markers from positive blood cultures and potential impact for more-rapid antibiotic interventions. J Clin Microbiol. 2014;52:3805–7. DOIPubMedGoogle Scholar
- Donà D, Di Chiara C, Sharland M. Multi-drug-resistant infections in the COVID-19 era: a framework for considering the potential impact. J Hosp Infect. 2020;106:198–9. DOIPubMedGoogle Scholar
- Kaier K, Mutters NT, Frank U. Bed occupancy rates and hospital-acquired infections—should beds be kept empty? Clin Microbiol Infect. 2012;18:941–5. DOIPubMedGoogle Scholar
- Getahun H, Smith I, Trivedi K, Paulin S, Balkhy HH. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull World Health Organ. 2020;98:442–442A. DOIPubMedGoogle Scholar
- Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–68.PubMedGoogle Scholar
- Vincent J-L, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, et al.; EPIC III Investigators. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020;323:1478–87. DOIPubMedGoogle Scholar
- Prestel C, Anderson E, Forsberg K, Lyman M, de Perio MA, Kuhar D, et al. Candida auris outbreak in a COVID-19 specialty care unit—Florida, July–August 2020. MMWR Morb Mortal Wkly Rep. 2021;70:56–7. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: February 10, 2021
Table of Contents – Volume 27, Number 4—April 2021
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Surbhi Leekha, University of Maryland School of Medicine, 10 South Pine St, MSTF 334F, Baltimore, MD 21201, USA
Top