Volume 27, Number 5—May 2021
Research
Clinical Evaluation of Roche SD Biosensor Rapid Antigen Test for SARS-CoV-2 in Municipal Health Service Testing Site, the Netherlands
Figure 2
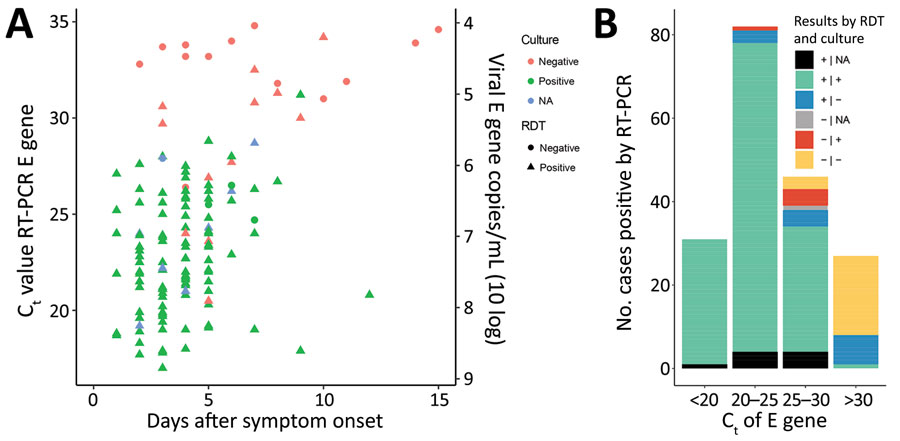
Figure 2. Relationships of time from symptom onset to testing and cycle threshold values to results for rapid antigen detection tests and PCR for diagnosis of severe acute respiratory syndrome coronavirus 2, the Netherlands. A) Cycle thresholds of positive samples in relation to days since symptom onset, Ag RDT positivity, and culture outcomes of participation with known disease onset date (n = 140). B) PCR-positive samples by cycle threshold (n = 186) in relation to Ag RDT and culture test results. Ag RDT, antigen rapid detection test; Ct, cycle threshold; E gene, envelope gene; NA, not available; RT-PCR, reverse transcription PCR.
Page created: March 16, 2021
Page updated: April 20, 2021
Page reviewed: April 20, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.