Volume 27, Number 9—September 2021
Research
Development and Clinical Evaluation of a CRISPR-Based Diagnostic for Rapid Group B Streptococcus Screening
Figure 1
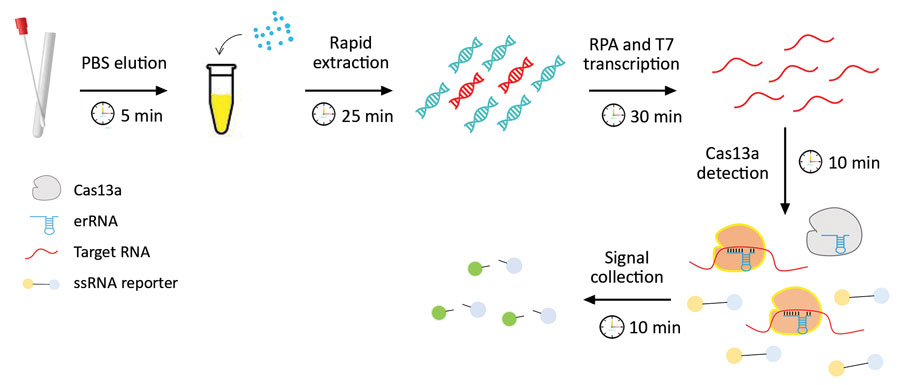
Figure 1. Schematic diagram of CRISPR-based diagnostic for rapid GBS screening. Swab samples are first eluted and followed by a rapid DNA extraction step where the bacterial cell walls are disrupted by a combination of chemical, physical, and heating effects. The extracted DNA is then subjected to the CRISPR/Cas reaction. The collateral nuclease activity of Cas proteins are activated upon specific binding of gRNA to the atoB gene. Fluorescent signal produced from cleaved probes is captured and indicates the presence of GBS. GBS, group B Streptococcus. gRNA, guide RNA; ssRNA, single-stranded RNA.
1These authors contributed equally to this article.
Page created: July 14, 2021
Page updated: August 25, 2021
Page reviewed: August 25, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.