Volume 27, Number 9—September 2021
Research
Development and Clinical Evaluation of a CRISPR-Based Diagnostic for Rapid Group B Streptococcus Screening
Figure 4
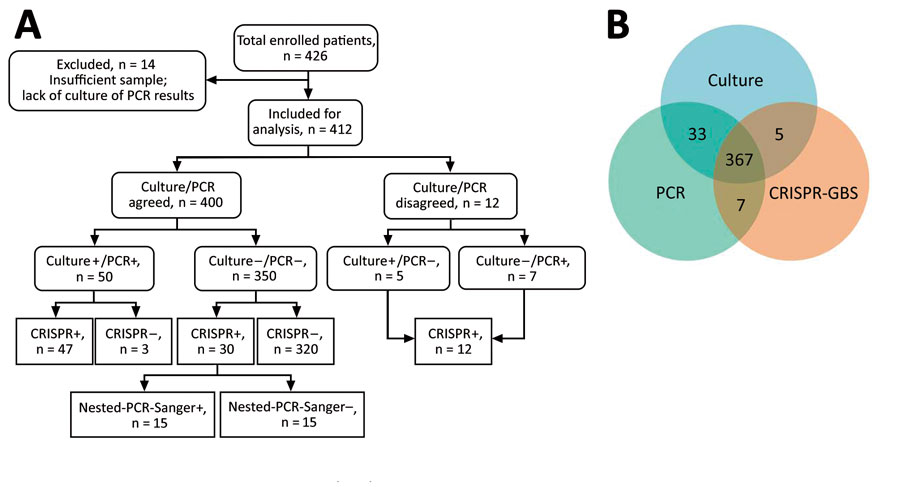
Figure 4. Overview and summary of the prospective cohort study assessing CRISPR-GBS. A) Study enrollment and result summary as categorized by agreements between different tests. B) Venn diagram demonstrating the overall concordance and discordance among direct culture, regular PCR, and CRISPR-GBS in the cohort. CRISPR-GBS, CRISPR-based diagnostic for rapid group B Streptococcus screening.
1These authors contributed equally to this article.
Page created: July 14, 2021
Page updated: August 25, 2021
Page reviewed: August 25, 2021
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.