Volume 28, Number 2—February 2022
Research Letter
Novel Anaplasmataceae agents Candidatus Ehrlichia hydrochoerus and Anaplasma spp. Infecting Capybaras, Brazil
Abstract
We amplified Ehrlichia and Anaplasma DNA from Amblyomma dubitatum tick–infested capybaras (Hydrochoerus hydrochaeris) in southern Brazil. Sequencing of 16S rRNA, sodB, and groEL indicated a novel Ehrlichia species, and sequencing of 16S rRNA from 2 capybaras indicated a novel Anaplasma species. The tick vectors remain unknown.
Ehrlichia and Anaplasma species are tickborne bacteria that infect animals and humans worldwide. To date, 6 Ehrlichia species have been described (E. canis, E. chaffeensis, E. ewingii, E. muris, E. ruminantium, and E. minasensis), and 8 Anaplasma species have been described (A. bovis, A. capra, A. centrale, A. marginale, A. odocoilei, A. ovis, A. platys, and A. phagocytophilum). In addition, other native Ehrlichia species have been described in wild animals from Brazil (1).
Although capybaras (Hydrochoerus hydrochaeris), the largest living rodents in the world, have been implicated as a major amplifying host of Rickettsia rickettsii (the etiologic agent of Brazilian spotted fever) for Amblyomma sculptum ticks, studies focusing on other tickborne diseases agents are lacking in this rodent. Accordingly, we conducted a comprehensive survey for the detection of Ehrlichia and Anaplasma species in a population of capybaras from Pinhais Municipality, Paraná State, southern Brazil.
We retrieved blood samples from 17 capybaras and salivary glands from 11 Amblyomma dubitatum ticks from these capybaras that were collected for a previous study conducted in southern Brazil (2). We screened blood samples by using PCR targeting of the 16S rRNA gene of Ehrlichia and Anaplasma (3,4). We then tested samples positive by PCR by using PCR that targeted a fragment of the dsb and sodB genes of Ehrlichia species (1,5) and the groEL gene of Ehrlichia and Anaplasma species (6). We used blood samples from dogs positive for E. canis as positive controls and nuclease-free water samples as negative controls.
The Ehrlichia 16S rRNA PCR assay yielded amplicons in 16/17 (94.12% [95% CI 73.02%–98.95%]) capybaras, from which we generated amplicons by the sodB PCR (300 bp) and groEL PCR (1,100 bp) assays. No sample yielded amplicon by the dsb PCR assay. We sequenced amplicons obtained from 4 16S rRNA, 5 sodB, and 4 groEL PCR-positive samples in both directions by using the Sanger method. We submitted all nucleotide sequences obtained to GenBank (Appendix).
We observed infestations by A. dubitatum ticks in all capybaras, from which we collected 26 males, 16 females, and 122 nymphs. Among salivary glands from 11 adult ticks, 1 (9.09%) tested positive for Ehrlichia species by the 16S rRNA PCR. However, multiple attempts to sequence the 16S rRNA gene detected in tick salivary glands were unsuccessful because of the faint bands.
We observed neither abnormalities nor inclusion-like bodies of Ehrlichia or Anaplasma during the evaluation of Giemsa-stained thin blood smears of the capybaras. We tested Ehrlichia antibodies in capybara serum samples with an indirect immunofluorescent assay using E. canis (São Paulo and Cuiabá strains) as antigens; serum samples were positive if reacting at a dilution >1:40 (7). A total of 6/17 (35.29% [95% CI 17.31%–58.70%]) capybaras showed antibodies against >1 of the E. canis antigens. When we used the Cuiabá strain of E. canis as antigen, 4/17 (23.53% [95% CI 9.56%–47.26%]) capybaras were seropositive, whereas 6/17 (35.29%) were positive when we used the São Paulo strain. Four capybaras were seropositive for both E. canis strains. Antibody endpoint titers ranged from 40 to 640 for both E. canis antigens.
According to serologic testing, PCR amplification, and DNA sequencing results, A. dubitatum tick–infested capybaras in southern Brazil may be infected with a novel Ehrlichia agent and a novel Anaplasma species. Serologic screening showed exposure to Ehrlichia species in 35% of the capybaras. A previous study failed to detect Ehrlichia DNA in spleen tissue of capybaras from southeastern Brazil (8), and we know of no previous study of Anaplasma species that has been performed in this rodent species.
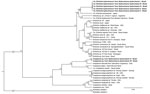
Partial sequences of 16S rRNA and 2 protein-coding genes (sodB and groEL) obtained from capybaras indicate a novel Ehrlichia species. Partial 16S rRNA gene sequences from capybara no. II showed that the detected Ehrlichia agent shared 95.67% identity with A. phagocytophilum, whereas sequences from capybara no. III showed that the detected Ehrlichia agent shared 94.28% identity with E. chaffeensis. Partial sodB genes showed 82.23%–85.07% identity with E. chaffeensis or E. ruminantium, whereas partial groEL genes showed identity with 76.52% with A. phagocytophilum. A previous study stated that different bacterial isolates showing <97% similarity in the 16S rRNA gene belong to different species (9). In addition, protein-coding genes should be used in addition to the 16S rRNA gene for identification of novel species (10). Our genetic findings support the infection of capybaras in Brazil with a novel Ehrlichia species, herein named Candidatus Ehrlichia hydrochoerus (Figure).
Partial sequences of 16S rRNA gene obtained from capybaras VI and VII demonstrated a novel Anaplasma species. Partial 16S rRNA gene sequences showed identity of 96.76% with Anaplasma sp. detected in dogs from the Philippines and 97.93% with A. phagocytophilum, with 100% query coverage. Bayesian inference showed that the capybara Anaplasma species detected was related to A. odocoilei from North America, which indicates a novel Anaplasma species infecting capybaras in Brazil.
Dr. Vieira is a research associate at the Vector-borne Diseases Laboratory, Universidade do Paraná, Curitiba, Brazil, and specializes in tickborne diseases. Her research is focused on molecular characterization of Anaplasmatacae in Brazil.
Acknowledgments
We thank Hubert D. Fanien for providing O’TOM/Tick Twister.
The Fundação Araucária (grant no. 09/2016) and the Brazilian National Council of Scientific and Technological Development (grant no. 425597/2018-0) provided financial aid and support to carry out this research. The Brazilian National Council of Scientific and Technological Development also provided research fellowships to D.M.A. (grant no. 303677/2018-0), M.B.L. (grant no. 301641/2019-6), and R.F.C.V. (grant no. 313161/2020-8). F.C.M.C. was sponsored by a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior at the time of this study.
References
- Almeida AP, Souza TD, Marcili A, Labruna MB. Novel Ehrlichia and Hepatozoon agents infecting the crab-eating fox (Cerdocyon thous) in southeastern Brazil. J Med Entomol. 2013;50:640–6. DOIPubMedGoogle Scholar
- Vieira RFC, Santos NJR, Valente JDM, Santos LP, Lange RR, Duque JCM, et al. ‘Candidatus Mycoplasma haematohydrochoerus’, a novel hemoplasma species in capybaras (Hydrochoerus hydrochaeris) from Brazil. Infect Genet Evol. 2021;93:
104988 . DOIPubMedGoogle Scholar - Parola P, Roux V, Camicas JL, Baradji I, Brouqui P, Raoult D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans R Soc Trop Med Hyg. 2000;94:707–8. DOIPubMedGoogle Scholar
- Ruiz-Fons F, Fernández-de-Mera IG, Acevedo P, Gortázar C, de la Fuente J. Factors driving the abundance of ixodes ricinus ticks and the prevalence of zoonotic I. ricinus-borne pathogens in natural foci. Appl Environ Microbiol. 2012;78:2669–76. DOIPubMedGoogle Scholar
- Qurollo BA, Davenport AC, Sherbert BM, Grindem CB, Birkenheuer AJ, Breitschwerdt EB. Infection with Panola Mountain Ehrlichia sp. in a dog with atypical lymphocytes and clonal T-cell expansion. J Vet Intern Med. 2013;27:1251–5. DOIPubMedGoogle Scholar
- Barber RM, Li Q, Diniz PP, Porter BF, Breitschwerdt EB, Claiborne MK, et al. Evaluation of brain tissue or cerebrospinal fluid with broadly reactive polymerase chain reaction for Ehrlichia, Anaplasma, spotted fever group Rickettsia, Bartonella, and Borrelia species in canine neurological diseases (109 cases). J Vet Intern Med. 2010;24:372–8. DOIPubMedGoogle Scholar
- Aguiar DM, Cavalcante GT, Pinter A, Gennari SM, Camargo LM, Labruna MB. Prevalence of Ehrlichia canis (Rickettsiales: Anaplasmataceae) in dogs and Rhipicephalus sanguineus (Acari: Ixodidae) ticks from Brazil. J Med Entomol. 2007;44:126–32. DOIPubMedGoogle Scholar
- Labruna MB, McBride JW, Camargo LM, Aguiar DM, Yabsley MJ, Davidson WR, et al. A preliminary investigation of Ehrlichia species in ticks, humans, dogs, and capybaras from Brazil. Vet Parasitol. 2007;143:189–95. DOIPubMedGoogle Scholar
- Drancourt M, Raoult D. Sequence-based identification of new bacteria: a proposition for creation of an orphan bacterium repository. J Clin Microbiol. 2005;43:4311–5. DOIPubMedGoogle Scholar
- Fournier PE, Dumler JS, Greub G, Zhang J, Wu Y, Raoult D. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J Clin Microbiol. 2003;41:5456–65. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: January 13, 2022
Table of Contents – Volume 28, Number 2—February 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Thállitha S.W.J. Vieira, Departamento de Medicina Veterinária, Campus Agrárias, Universidade Federal do Paraná. R. dos Funcionários, 1540, Juvevê, Curitiba, PR, 80035-050, Brazil
Top