Volume 28, Number 3—March 2022
Research Letter
SARS-CoV-2 Breakthrough Infections after introduction of 4 COVID-19 Vaccines, South Korea, 2021
Cite This Article
Citation for Media
Abstract
We conducted a nationwide retrospective cohort study to estimate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) breakthrough infection among recipients of 4 different vaccines in South Korea. Age-adjusted breakthrough infection rate per month was highest for Janssen (42.6/100,000 population), followed by AstraZeneca (21.7/100,000 population), Pfizer-BioNTech (8.5/100,000 population), and Moderna (1.8/100,000 population).
Since their rollout, vaccines have been highly effective globally in controlling coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). Breakthrough infections have been reported in some vaccine recipients, suggesting the need for public health assessment and monitoring (2). To date, the vaccine-specific data on breakthrough infections are limited. In early 2021, the national immunization program of South Korea introduced 4 COVID-19 vaccines: ChAdOx1 nCov-19 (AstraZeneca, https://www.astrazeneca.com), BNT162b2 (Pfizer-BioNTech, https://www.pfizer.com), Ad26.COV2.S (Johnson & Johnson/Janssen [hereafter Janssen], https://www.janssen.com), and mRNA-1273 (Moderna, https://www.moderna.com). As of October 10, 2021, a total of 70% of the country’s population have received ≥1 dose of vaccine (3). Introduction of the vaccines provided an opportunity to study breakthrough infections by different vaccine types. We describe a snapshot of SARS-CoV-2 breakthrough infections in South Korea and aim to identify risk by age group that might influence the observed pattern.
We conducted a nationwide retrospective cohort study to estimate SARS-CoV-2 breakthrough infection rates among AstraZeneca, Pfizer-BioNTech, Janssen, and Moderna vaccine recipients in South Korea. We included fully vaccinated persons (2 weeks past 2-dose vaccination for AstraZeneca, Pfizer-BioNTech, and Moderna vaccines; 2 weeks past 1-dose vaccination for Janssen vaccine) without history of SARS-CoV-2 infection (Appendix Figure 1). A Pfizer-BioNTech booster vaccination was offered to AstraZeneca vaccine–primed persons (2 doses of AstraZeneca vaccine, then a third dose of Pfizer-BioNTech vaccine), who were thereafter included in the analysis. Observed periods were April 7–October 10, 2021, for AstraZeneca vaccine; April 3–October 10, 2021, for Pfizer-BioNTech vaccine; June 24–October 10, 2021, for Janssen vaccine; July 30–October 10, 2021, for Moderna vaccine; and July 19–October 10, 2021, for AstraZeneca/Pfizer-BioNTech prime/booster recipients.
We estimated breakthrough infection rate by vaccine, number of serious outcomes (cases treated with high-flow oxygen therapy, mechanical ventilator, extracorporeal membrane oxygenation, continuous renal replacement therapy, or death), and number of secondary transmissions originated from the breakthrough infection case. We identified the presence of serious outcomes through the case reporting form collected under the Infectious Disease Control and Prevention Act, which mandates epidemiologic investigation on all confirmed SARS-CoV-2 cases in South Korea. In all close contacts of laboratory-confirmed SARS-CoV-2 case-patients, we conducted epidemiologic investigations to search for the preceding link and potential onward transmission cases. We calculated age-adjusted and age-specific breakthrough infection rates as well as age-adjusted rates for serious outcomes and deaths. We randomly tested ≈20% of samples for full-length genome and spike protein sequencing to identify the presence of variant of concern.
The number of vaccinations by vaccine type are as follows: AstraZeneca (prime/booster), 8,737,343 persons; Pfizer-BioNTech (prime/booster), 10,235,891 persons; Janssen (single), 1,408,921 persons; Moderna (prime/booster), 1,190,973 persons; and AstraZeneca/Pfizer-BioNTech (prime/booster), 1,600,998 persons (Table). Age-adjusted breakthrough infection rate per month was highest among Ad26.COV2.S recipients (42.6/100,000 population), followed by AstraZeneca (prime/booster) recipients (21.7/100,000 population), AstraZeneca/Pfizer-BioNTech (prime/booster) recipients (21.3/100,000 population), Pfizer-BioNTech (prime/booster) recipients (8.5/100,000 population), and Moderna (prime/booster) recipients (1.8/100,000 population). Serious outcome (0–0.9/100,000 population) and death (0–0.2/100,000 population) after breakthrough infection were rare for all vaccine types. Secondary transmission rate was highest among Janssen recipients (19.2/100,000 population), followed by AstraZeneca (prime/booster) recipients (4.9/100,000 population).
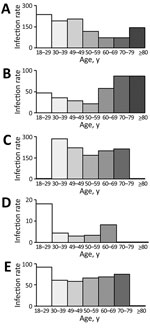
The highest breakthrough infection rates we observed in younger age groups were in AstraZeneca (prime/booster), Janssen (single), Moderna (prime/booster), and AstraZeneca/Pfizer-BioNTech (prime/booster) recipients (Figure). Among the Pfizer-BioNTech (prime/booster) recipients, breakthrough infection rate was highest among elderly persons 70–79 years and ≥80 years of age (Appendix Figure 2).
We identified the variants of concern found in AstraZeneca (prime/booster) recipients as 1,285 Delta and 4 Alpha variants; in Pfizer-BioNTech (prime/booster) as 888 Delta, 14 Alpha, and 1 Beta variants; in Janssen (single), 789 Delta, 12 Alpha, and 2 Gamma variants; in Moderna (prime/booster), 13 Delta variants; and in AstraZeneca/Pfizer-BioNTech (prime/booster), 188 Delta variants.
Our findings of a higher breakthrough infection in adenovirus DNA vector vaccine recipients and lower risk among mRNA vaccine recipients are consistent with other studies. In clinical trials, 0.5% of AstraZeneca recipients (4) and 0.3% of Janssen recipients (5) had SARS-CoV-2 infections, whereas 0.05% of Pfizer-BioNTech recipients (6) and 0.08% of Moderna recipients (7) had infections. The AstraZeneca/Pfizer-BioNTech (prime/booster) recipients had breakthrough infection rate in between that of AstraZeneca (prime/booster) and Pfizer-BioNTech (prime/booster) recipients, suggesting a potential benefit from mix-and-match vaccination as observed in previous studies (8).
A limitation of this study is that the observed period between the vaccines were different: AstraZeneca and Pfizer-BioNTech were available for nearly 6 months, whereas Janssen and Moderna were introduced 2–3 months later. We conducted monthly adjustments of daily data; however, unidentified confounders may have affected the observed result. In addition, emergence of new variants may also affect the risk for breakthrough infection (9). Since mid-June 2021, Delta variant has become the dominant strain in South Korea, which may have affected vaccine effectiveness and postinfection health outcomes. Despite these limitations, our findings demonstrate uniformly low numbers of serious disease cases in recipients of all 4 vaccine types, consistent with previous findings (10).
In conclusion, breakthrough infection was more common among adenovirus DNA vector vaccine recipients than among mRNA vaccine recipients. Booster vaccination with mRNA vaccines in adenovirus DNA vector vaccine–primed individuals may confer additional protection against SARS-CoV-2 breakthrough infections.
Dr. Yi is a public health officer at Korea Disease Control and Prevention Agency. Her main research interest is in epidemiologic investigation and surveillance measures of infectious diseases. Dr. Choe is a clinical assistant professor of pediatrics at Korea University Anam Hospital. His main research addresses quantification of and understanding the mechanisms of immunization program's impact on public health.
Acknowledgment
We thank the relevant ministries, including the Ministry of Interior and Safety, Si/Do and Si/Gun/Gu, medical staffs in health centers, and medical facilities for their efforts in responding to COVID-19 outbreak.
References
- Thompson MG, Stenehjem E, Grannis S, Ball SW, Naleway AL, Ong TC, et al. Effectiveness of Covid-19 Vaccines in Ambulatory and Inpatient Care Settings. N Engl J Med. 2021;385:1355–71. DOIPubMedGoogle Scholar
- Scobie HM, Johnson AG, Suthar AB, Severson R, Alden NB, Balter S, et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status - 13 U.S. Jurisdictions, April 4-July 17, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1284–90. DOIPubMedGoogle Scholar
- Korea Disease Control and Prevention Agency. COVID-19 vaccination dashboard [cited 2021 Oct 25]. https://ncv.kdca.go.kr/eng/
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al.; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. DOIPubMedGoogle Scholar
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al.; ENSEMBLE Study Group. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187–201. DOIPubMedGoogle Scholar
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al.; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–15. DOIPubMedGoogle Scholar
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al.; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–16. DOIPubMedGoogle Scholar
- Tenbusch M, Schumacher S, Vogel E, Priller A, Held J, Steininger P, et al.; DZIF-VACCELERATE-CoVaKo study team. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis. 2021;21:1212–3. DOIPubMedGoogle Scholar
- Blanquart F, Abad C, Ambroise J, Bernard M, Cosentino G, Giannoli J-M, et al. Characterisation of vaccine breakthrough infections of SARS-CoV-2 Delta and Alpha variants and within-host viral load dynamics in the community, France, June to July 2021. Euro Surveill. 2021;26. DOIPubMedGoogle Scholar
- Butt AA, Yan P, Shaikh OS, Mayr FB. Outcomes among patients with breakthrough SARS-CoV-2 infection after vaccination in a high-risk national population. EClinicalMedicine. 2021;40:
101117 . DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: February 08, 2022
1These authors contributed equally to this article.
Table of Contents – Volume 28, Number 3—March 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Young-Joon Park, Director of Epidemiologic Investigation. Korea Disease Control and Prevention Agency, Cheongju, South Korea
Top