Volume 28, Number 4—April 2022
Research
Fatal Human Alphaherpesvirus 1 Infection in Free-Ranging Black-Tufted Marmosets in Anthropized Environments, Brazil, 2012–2019
Figure 3
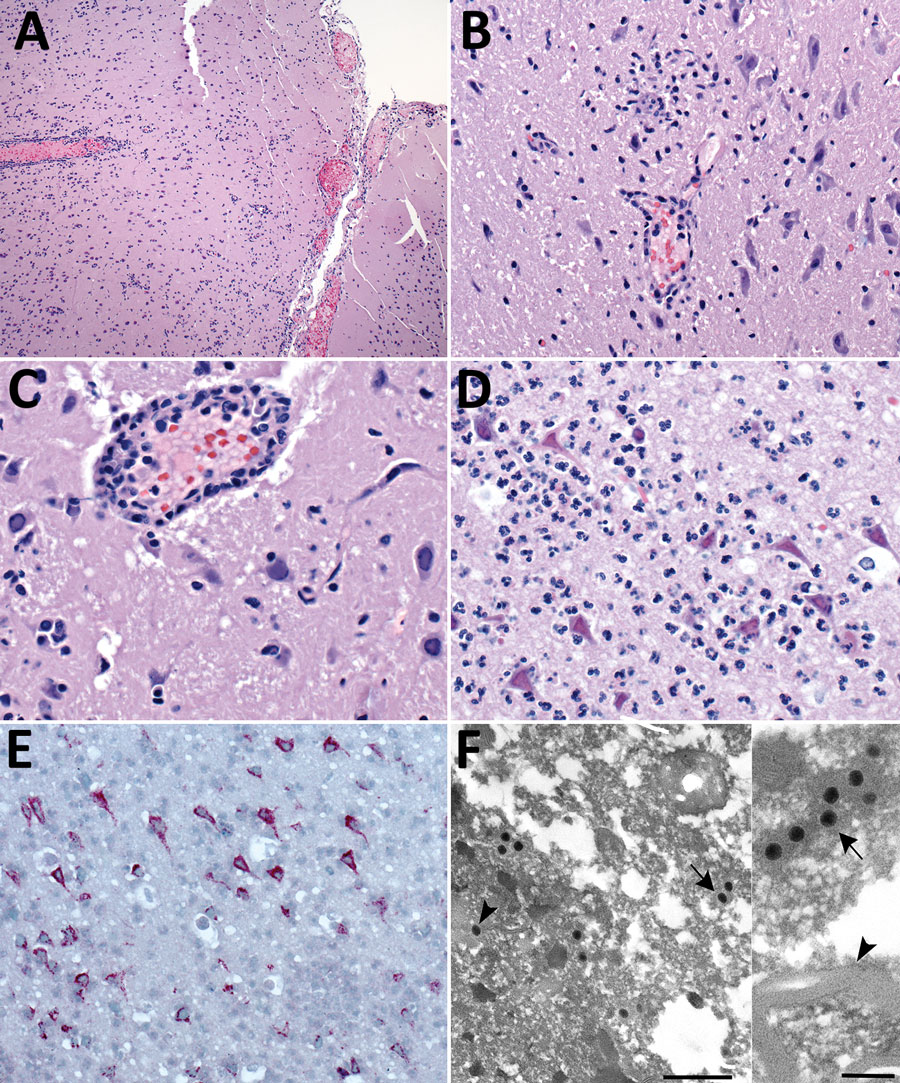
Figure 3. Pathologic changes in brain of free-ranging black-tufted marmosets with fatal human alphaherpesvirus 1 infection, Brazil, 2012–2019. A) Necrotizing meningoencephalitis. Hemotoxylin and eosin (H&E) stain; original magnification ×10. B) Neuronal degeneration and glial nodule. H&E stain; original magnification ×40. C) Neuronal necrosis with microglial proliferation and expansion of Virchow–Robbin spaces by lymphocytes, histiocytes, and few plasma cells. Neurons and glial cells show intranuclear inclusion bodies and prominent margination of the nuclear chromatin. H&E stain; original magnification ×63. D) Prominent neutrophilic inflammation accompanies neuronal necrosis and intranuclear inclusion bodies. H&E stain; original magnification ×63. E) Human alphaherpesvirus 1 immunostaining within neurons (immunohistochemistry; original magnification ×40). F) Intranuclear (arrowhead) and cytoplasmic (arrow) herpesvirus particles in gray matter. Transmission electron microscopy; scale bar indicate 500 nm. Inset: cytoplasmic herpesvirus particles (arrow) white matter, myelinated axon (arrowhead); scale bar indicates 200 nm.