Volume 28, Number 9—September 2022
Research Letter
Trichodysplasia Spinulosa Polyomavirus Endothelial Infection, California, USA
Cite This Article
Citation for Media
Abstract
We describe 3 patients in California, USA, with trichodysplasia spinulosa polyomavirus (TSPyV) infection of endothelium after steroid administration. We detected TSPyV RNA in tissue specimens by in situ hybridization, which revealed localization to endothelial cells. These cases suggest that diseases associated with endothelial inflammation could be associated with TSPyV infection.
Trichodysplasia spinulosa polyomavirus (TSPyV) is an alphapolyomavirus whose primary clinical manifestation in a posttransplant setting is folliculocentric papular cutaneous eruptions, typically involving the face (1). Identification of TSPyV nucleic acids in tonsillar tissue has led to the speculation that lymphoid tissue might be a latency site (2); however, some disagreement exists in the literature as to whether the clinical diagnosis of trichodysplasia spinulosa reflects primary infection or reactivation of latent virus (3,4). Although cutaneous disease is the primary clinical manifestation of infection, TSPyV has been identified in blood, urine, cerebrospinal fluid, tonsils, and respiratory specimens by various methods, including nucleic acid detection, immunohistochemistry, and electron microscopy (2,4–6). TSPyV DNA loads can be high, especially in blood (up to 108 viral copies/mL), months before the appearance of typical trichodysplasia spinulosa skin lesions (4).
This case study was part of a larger project approved by the Stanford Institutional Review Board (approval no. 58311) designed to explore oncogenesis by alphapolyomaviruses. We identified rare cases on our next generation sequencing panel of solid tumors with off-target, high quality reads that aligned to the TSPyV genome. We hypothesized that some of these cases might represent TSPyV-mediated neoplasms. Our cases comprised 1 patient with metastatic lung adenocarcinoma involving the brain (case 1), 1 patient with meningioma (case 2), and 1 patient with a metastatic perivascular epithelial cell tumor involving the liver (Table). It is unclear whether the mental status changes observed in cases 1 and 2 were attributable to viral infection or were secondary to the tumors (Table). All 3 patients received steroids immediately preceding resection. We performed in situ hybridization using a custom RNAScope probe that targeted the complete TSPyV viral genome (GenBank accession no. NC_014361.1) and RNAscope 2.5 HD Reagent Kit-RED (Advanced Cell Diagnostics, https://acdbio.com) to detect TSPyV RNA in cutaneous biopsy specimens. One case of cutaneous trichodysplasia spinulosa was used as a positive control for TSPyV RNA. We used the RNAscope 2.5 LS Probe-Hs-PPIB-sense probe (Advanced Cell Diagnostics), which is specific for peptidylprolyl isomerase B, to demonstrate the presence of intact RNA in the tissue sections. For negative in situ hybridization controls, we used tissue specimens from patients with Merkel cell polyomavirus-positive Merkel cell carcinoma and BK polyomavirus nephropathy and a T-cell lymphoma tissue microarray that included 1 case of Merkel cell polyomavirus-positive T-cell lymphoma.
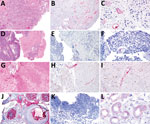
In contrast to our hypothesis, we found that TSPyV RNA did not localize to neoplastic cells. However, TSPyV RNA localized to the endothelium in all 3 cases (Figure). The BK polyomavirus nephropathy sections showed weak, orange-red discoloration of tubular epithelium (Figure, panel L) that was distinguishable from the bright red, granular staining patterns for TSPyV RNA observed in the positive control and tissue specimens from the 3 patients. This discoloration was interpreted as negative for TSPyV RNA by board-certified pathologists who had experience evaluating RNAScope in situ hybridizations.
TSPyV is a DNA virus, and detection of TSPyV RNA indicates active viral replication and infection. Our in situ hybridization results suggest that TSPyV can infect endothelial cells, likely within various tissues. These cases provide insight into a potential cellular reservoir for TSPyV infection. In addition, these data raise the possibility that other diseases associated with endothelial inflammation could be associated with TSPyV infection. Overall, this small case series improves our knowledge of the scope of human TSPyV infection.
Dr. Lawrence is a molecular genetic pathology fellow at Stanford Healthcare in the Department of Pathology. Her clinical and research interests focus on molecular diagnostics and hematopathology.
Acknowledgments
We thank Norm Cyr for assistance with the figure layout.
This study was supported by a Mentored Trainee Grant for Personalized Medicine in the Department of Pathology, Stanford University.
References
- Narayanan D, Rady PL, Tyring SK. Recent developments in trichodysplasia spinulosa disease. Transpl Infect Dis. 2020;22:
e13434 . DOIPubMedGoogle Scholar - Sadeghi M, Aaltonen LM, Hedman L, Chen T, Söderlund-Venermo M, Hedman K. Detection of TS polyomavirus DNA in tonsillar tissues of children and adults: evidence for site of viral latency. J Clin Virol. 2014;59:55–8. DOIPubMedGoogle Scholar
- Wu JH, Nguyen HP, Rady PL, Tyring SK. Molecular insight into the viral biology and clinical features of trichodysplasia spinulosa. Br J Dermatol. 2016;174:490–8. DOIPubMedGoogle Scholar
- van der Meijden E, Horváth B, Nijland M, de Vries K, Rácz EK, Diercks GF, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080–4.PubMedGoogle Scholar
- Borgogna C, Albertini S, Zavattaro E, Veronese F, Peruzzi L, van der Meijden E, et al. Primary trichodysplasia spinulosa polyomavirus infection in a kidney transplant child displaying virus-infected decoy cells in the urine. J Med Virol. 2019;91:1896–900. DOIPubMedGoogle Scholar
- Siebrasse EA, Bauer I, Holtz LR, Le BM, Lassa-Claxton S, Canter C, et al. Human polyomaviruses in children undergoing transplantation, United States, 2008-2010. Emerg Infect Dis. 2012;18:1676–9. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: August 10, 2022
1Current affiliation: Guardant Health, Redwood City, California, USA.
Table of Contents – Volume 28, Number 9—September 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Lauren Lawrence, Guardant Health, 505 Penobscot Dr, Redwood City, CA 94063, USA
Top