Volume 29, Number 1—January 2023
Dispatch
Pathologic and Immunohistochemical Evidence of Possible Francisellaceae among Aborted Ovine Fetuses, Uruguay
Abstract
The only genus of the Francisellaceae family known to contain species pathogenic to mammals is Francisella, for which reported cases in the Southern Hemisphere have been limited to Australia. We describe severe necrotizing and inflammatory lesions and intralesional immunohistochemical identification of Francisella sp. lipopolysaccharide among aborted ovine fetuses in Uruguay.
The Francisellaceae family comprises gram-negative coccobacilli and 4 genera are currently recognized: Francisella, Allofrancisella, Pseudofrancisella, and Cysteiniphilum (1), of which only Francisella is of clinical relevance. Francisella tularensis is the most studied species because it causes tularemia, a highly transmissible, potentially life-threatening, zoonotic disease, also considered a potential bioterrorism agent (2,3).
Tularemia occurs over almost the entire Northern Hemisphere but is rarely reported in the Southern Hemisphere, where the only published cases have occurred in Australia (4–7). F. tularensis comprises 4 subspecies, tularensis, holarctica, mediasiatica, and novicida. F. tularensis subsp. tularensis occurs almost exclusively in North America and is responsible for 80%–90% of the tularemia cases, despite the co-existence of subspecies holarctica, which is the cause of most tularemia cases in Europe (2). The few cases of F. tularensis infection described in Australia were associated with F. tularensis subsp. holarctica and novicida (4–6). In the Americas, tularemia occurs in the United States, Mexico, and Canada (2,8), and no disease caused by Francisella spp. bacteria in mammals has been reported south of Mexico.
Although F. tularensis has a broad host range, sheep are the only livestock species affected by epizootics of tularemia and have been implicated in disease transmission to humans (9,10). We report a case of ovine abortion in Uruguay that raises concerns about the possible occurrence of tularemia in South America.
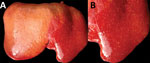
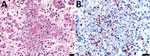
In July 2015, two (≈1%) of ≈200 pastured sheep on a family farm in Colonia, Uruguay, aborted at ≈4 months of gestation. Autopsies on twin aborted fetuses (A and B) showed similar gross lesions (Table 1), consisting of severe multifocal widespread necrotizing hepatitis (Figure 1), and moderate fibrinous peritonitis and pericarditis. Samples of liver, adrenal gland, spleen, lung, heart, kidney, and brain tissues of both fetuses were fixed in formalin, then processed, embedded in paraffin, microtome-sectioned, and stained with hematoxylin and eosin. Histopathologic examination revealed severe acute multifocal random fibrinonecrotizing neutrophilic and histiocytic hepatitis (Figure 2, panel A), multifocal necrotizing and neutrophilic myocarditis, multifocal neutrophilic bronchiolitis and alveolitis, and multifocal fibrinous splenic capsulitis.
We processed formalin-fixed paraffin-embedded sections of liver from fetus B for immunohistochemistry to detect Francisella antigen (Appendix). We used tissue from a squirrel with culture- and PCR-confirmed F. tularensis septicemia as a positive control. We performed antigen retrieval in a decloaking chamber by using Antigen Decloaker citrate buffer (Biocare Medical, https://biocare.net). We applied a specific mouse monoclonal IgG3 raised against F. tularensis lipopolysaccharide, F. tularensis LPS Monoclonal Antibody (T14) (Thermo Fisher Scientific, https://www.thermofisher.com) as the primary antibody at 1:1,000 dilution. We used Mouse-on-Farma HRP-Polymer (Biocare Medical) and 3-amino-9-ethylcarbazole (Thermo Fisher Scientific) for antigen detection. For negative controls, we replaced F. tularensis monoclonal antibody with normal mouse IgG for both ovine and squirrel reactions.
Immunohistochemistry revealed strong abundant intralesional granular immunoreactivity in the necrotic foci of the fetal ovine liver, which was largely intracytoplasmic in infiltrating neutrophils and macrophages (Figure 2, panel B). We observed immunoreactivity in the positive control squirrel tissue but not in the negative controls. We conducted ancillary testing to rule out other ovine abortifacients (Table 2).
We postfixed formalin-fixed sections of liver from fetus B in modified Karnovsky’s fixative, 1% osmium tetroxide, and 0.1 mol cacodylate buffer, then processed and embedded sections in resin for transmission electron microscopy. Despite suboptimal ultrastructural tissue preservation due to autolysis, intrahistiocytic and extracellular ≈0.7–1.7 μm gram-negative coccobacilli colocalized with the foci of necrotizing hepatitis.
The lack of historical reports of tularemia outside endemic areas of North America and Eurasia has been puzzling (6). Recently, tularemia emerged in Australia (3) and reemerged in the Northern Hemisphere (7). South America has been considered free of tularemia (7); a status that seems to be based solely on the lack of disease reporting. However, tularemia might have been undiagnosed because of limitations in disease surveillance systems in the region. No clinical disease caused by Francisella spp. in mammals in the Americas south of Mexico has been described. Our results raise concerns about the possible occurrence of tularemia in South America.
The abortifacient effects of F. tularensis in sheep have been described in the United States, and tularemia has been regarded as an overlooked syndrome in sheep (9). From a pathologic viewpoint, necrotic foci in the liver, spleen, or lungs in late term aborted ovine fetuses are characteristic of tularemia and should raise suspicion, although gross lesions can be absent even in cases with typical histologic inflammatory and necrotizing lesions (9). Contrary to most bacterial abortifacients of sheep (11), F. tularensis is not visible upon histopathologic examination of tissues stained with hematoxylin and eosin, Steiner silver, or Gram stains, even in tissues that have a high bacterial burden demonstrated by immunohistochemistry (9). The ultrastructural demonstration of intracellular gram-negative coccobacilli of the expected size in phagocytic and inflammatory cells in tissues with lesions, as in our case, aids in the diagnosis. Diagnostic investigation of any case of ovine abortion with fetal lesions indicating a bacterial etiology should include ancillary testing to identify F. tularensis and rule out other abortigenic pathogens (11).
The etiologic diagnosis in our case was reached by the immunohistochemical demonstration of abundant intralesional antigen by a specific monoclonal antibody raised against F. tularensis lipopolysaccharide. Immunohistochemistry has proven useful for identifying F. tularensis in diagnostic settings (9,12). F. tularensis lipopolysaccharide is a main specific antigen and virulence factor and differs from the lipopolysaccharide of other gram-negative bacteria (13). According to the manufacturer, the primary antibody we used for immunohistochemistry does not cross-react with F. tularensis subsp. novicida, Yersinia pestis, Y. pseudotuberculosis, Y. enterocolitica, Vibrio cholerae, Escherichia coli, Salmonella enterica serovar Typhimurium, Brucella abortus, B. suis, B. ovis, B. melitensis, or B. neotomae. We tested the immunohistochemistry in cases of abortion caused by Campylobacter jejuni and C. fetus but observed no cross-reactivity. Although cross-reaction with other members of Francisellaceae cannot be completely ruled out, F. tularensis is currently the only species of this family recognized as an ovine abortifacient. Definite species and subspecies identification requires bacterial isolation and DNA analysis, which we were unable to perform because the available specimens were unsuitable.
Sheep with tularemia have been implicated in disease transmission to sheep industry workers (10). In the case described here, the owners of the sheep lived on the farm and were in contact with the affected flock regularly; however, we do not know whether they had clinical signs consistent with tularemia.
The source of infection in this sheep remained unknown. However, F. tularensis has a broad animal reservoir, including arthropods, rodents, lagomorphs, and marsupials (6,14). Brown hares (Lepus europaeus), a species that plays a primary role in the ecology of tularemia in Europe (12), have been introduced to Uruguay and are frequently seen around the affected farm. In addition, F. tularensis can be transmitted by ticks, several of which, including Amblyomma spp., Haemophysalis spp., and Ixodes spp. ticks, are endemic in Uruguay. Of note, a gamma-proteobacterium related to Francisella-like organisms, but different from F. tularensis, was identified in Uruguay in Amblyomma triste ticks (15), the most prevalent tick species reported in human tick bites in the country.
We provide pathologic and immunohistochemical evidence of disease caused by a possible Francisellaceae member in sheep in Uruguay. Additional research is needed to isolate and speciate the pathogen and elucidate its regional epidemiology. Nonetheless, veterinarians, physicians, and public health officials should be aware of possible tularemia in South America.
Dr. Giannitti is a principal investigator in veterinary pathology at the Instituto Nacional de Investigación Agropecuaria (INIA), La Estanzuela, Colonia, Uruguay. His primary research interests include the diagnostic investigation of naturally occurring diseases of livestock and wildlife and infectious abortifacients of ruminants.
Acknowledgments
We thank Yisell Perdomo for technical assistance with the histologic techniques.
This work was funded by research grant no. PL_27 N-23398 from the Instituto Nacional de Investigación Agropecuaria (INIA), Uruguay. M.A.D., R.D.C., and C.O.S. received financial support from INIA through graduate scholarships. R.D.C. also received financial support from the Agencia Nacional de Investigación e Innovación” (ANII), Uruguay, through a graduate scholarship.
References
- Parte AC, Sardà Carbasse J, Meier-Kolthoff JP, Reimer LC, Göker M. List of Prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. [cited 2022 Sep 21]. Int J Syst Evol Microbiol. 2020;70:5607–12. DOIPubMedGoogle Scholar
- Colquhoum DJ, Larsson P, Duodu S, Forsman M. Chapter 14: the family Francisellaceae. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes gammaproteobacteria, 4th ed. New York: Springer; 2014. p. 287–314.
- Maurin M. Francisella tularensis as a potential agent of bioterrorism? Expert Rev Anti Infect Ther. 2015;13:141–4. DOIPubMedGoogle Scholar
- Whipp MJ, Davis JM, Lum G, de Boer J, Zhou Y, Bearden SW, et al. Characterization of a novicida-like subspecies of Francisella tularensis isolated in Australia. J Med Microbiol. 2003;52:839–42. DOIPubMedGoogle Scholar
- Jackson J, McGregor A, Cooley L, Ng J, Brown M, Ong CW, et al. Francisella tularensis subspecies holarctica, Tasmania, Australia, 2011. Emerg Infect Dis. 2012;18:1484–6. DOIPubMedGoogle Scholar
- Eden JS, Rose K, Ng J, Shi M, Wang Q, Sintchenko V, et al. Francisella tularensis ssp. holarctica in Ringtail Possums, Australia. Emerg Infect Dis. 2017;23:1198–201. DOIPubMedGoogle Scholar
- Yeni DK, Büyük F, Ashraf A, Shah MSUD. Tularemia: a re-emerging tick-borne infectious disease. Folia Microbiol (Praha). 2021;66:1–14. DOIPubMedGoogle Scholar
- Lupi O, Madkan V, Tyring SK. Tropical dermatology: bacterial tropical diseases. J Am Acad Dermatol. 2006;54:559–78, quiz 578–80. DOIPubMedGoogle Scholar
- O’Toole D, Williams ES, Woods LW, Mills K, Boerger-Fields A, Montgomery DL, et al. Tularemia in range sheep: an overlooked syndrome? J Vet Diagn Invest. 2008;20:508–13. DOIPubMedGoogle Scholar
- Jellison WL, Kohls GM. Tularemia in sheep and in sheep industry workers in western United States. Public Health Monogr. 1955;28:1–19. DOIPubMedGoogle Scholar
- Dorsch MA, Cantón GJ, Driemeier D, Anderson ML, Moeller RB, Giannitti F. Bacterial, protozoal and viral abortions in sheep and goats in South America: a review. Small Rumin Res. 2021;205:
106547 . DOIGoogle Scholar - Gyuranecz M, Szeredi L, Makrai L, Fodor L, Mészáros AR, Szépe B, et al. Tularemia of European Brown Hare (Lepus europaeus): a pathological, histopathological, and immunohistochemical study. Vet Pathol. 2010;47:958–63. DOIPubMedGoogle Scholar
- Okan NA, Kasper DL. The atypical lipopolysaccharide of Francisella. Carbohydr Res. 2013;378:79–83. DOIPubMedGoogle Scholar
- Sjöstedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci. 2007;1105:1–29. DOIPubMedGoogle Scholar
- Venzal JM, Estrada-Peña A, Portillo A, Mangold AJ, Castro O, de Souza CG, et al. Detection of Alpha and Gamma-Proteobacteria in Amblyomma triste (Acari: Ixodidae) from Uruguay. Exp Appl Acarol. 2008;44:49–56. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: December 08, 2022
Table of Contents – Volume 29, Number 1—January 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Federico Giannitti, Plataforma de Investigación en Salud Animal, Instituto Nacional de Investigación Agropecuaria, Rte no. 50, Km. no. 11, 70006 La Estanzuela, Colonia, Uruguay
Top