Volume 29, Number 12—December 2023
Dispatch
Neurotropic Highly Pathogenic Avian Influenza A(H5N1) Virus in Red Foxes, Northern Germany
Abstract
In a 1-year survey of wild terrestrial predators in northern Germany, we found that 5 of 110 foxes were infected with contemporary avian influenza A(H5N1) viruses, forming a temporal cluster during January‒March 2023. Encephalitis and strong cerebral virus replication but only sporadic mammalian-adaptive viral polymerase basic 2 protein E627K mutations were seen.
Since emergence of the highly pathogenic avian influenza virus (HPAIV) H5 A/Goose/Guangdong/1/1996 (gs/GD) lineage in 1996, successors continue to circulate in waves around the world, leading to massive losses in wild bird and domestic poultry populations (1). Until 2020‒2021, gs/GD HPAIV infections in poultry holdings characteristically paralleled waterfowl migration patterns. Since then, this seasonality has virtually disappeared and gs/GD HPAIV, currently of subtype H5N1 assigned to clade 2.3.4.4b, are detected year-round in wild birds and poultry in Europe (2,3). The virus has been found at increasing frequency in domestic and wild living mammals, mostly affecting carnivorous species (4) and massive die-off events raised concern about potential mammal-to-mammal transmission in dense populations (5,6). HPAIV infections were regularly characterized by high viral loads in the brain and associated clinical signs of the central nervous system with corresponding morphologic changes (7–11). Although Germany has had high HPAIV infection rates in avian species, prevalence studies on HPAIV infections in terrestrial predators, which feed on (infected) waterfowl, are not available. We performed a 1 year-survey to detect HPAIV in wild terrestrial predators in northern Germany.
We studied HPAIV infections in 170 wildlife predators of several animal species: red foxes (Vulpes vulpes, n = 110), racoons (Procyon lotor, n = 28), badgers (Meles meles, n = 15), martens (Martes foina or Martes martes, n = 9), and racoon dogs (Nyctereutes procyonoides, n = 8) by using PCR and in situ methods. We performed avian influenza virus real-time PCR on individual brain samples of those 170 terrestrial wildlife predators and an H5-specific assay. The carcasses originated from different geographic locations in the German federal state of Lower Saxony and were delivered to the Lower Saxony State Office for Consumer Protection and Food Safety during February 2022–April 2023 (Appendix Table 1).
We detected viral RNA with cycle threshold values of 13.75–36.35 in the brains of 5 red foxes (4.5%), which were submitted with differing preliminary reports partly involving signs of disease specific for the central nervous system. Virus-positive animals were found in the first 3 months of 2023 (Table). In 4 of 5 cases, full-length influenza virus sequences could be recovered by using Illumina (https://www.illumina.com) high-throughput sequencing and were deposited in GISAID (https://gisaid.org) (Appendix Table 2). The hemagglutinin sequences of the 4 red foxes had nucleotide identities of 98.53%–99.06% and had a cleavage site typical for a highly pathogenic phenotype (REKRRKRG).
For the phylogenetic analyses, we included avian influenza virus nucleotide sequences representing the first 3 BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) search hits, as well as sequences detected in mammals during 2021‒2023 in Europe and relevant HPAIV H5N1 sequences recovered from wild birds from northern Germany. The phylogenetic tree showed that the H5 sequences detected in red foxes in this study belonged to clade 2.3.4.4b. They were closely related to sequences previously found in avian and mammal hosts in Europe but did not form a separate cluster (Figure 1). Analyses of all 8 genome segments assigned the genomes to genotype Ger-10–21-N1.5, Ger-11-21-N1.4, of Ger-02-23-N1.1 (Appendix Figure) (12). We further analyzed viral RNA segments for the presence of mutations, which have been described in mammalian H5Nx infections, involving amino acid residues in the polymerase basic (PB) 2 segment (7,9,11). The PB2 E627K substitution was found in the consensus sequence in only 1 of 4 cases (animal no. 51023–113) (Table).
HPAIV H5N1 isolates were obtained from 3 of 4 central nervous system tissue homogenates during the first passage in MDCK II cells. Hemagglutinating activity and high viral loads (cycle threshold values <20) were evident in the supernatant of inoculated cell cultures. An influenza-like cytopathic effect proceeded to affect the whole cell monolayer within less than 72 hours postinoculation (Table).
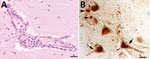
None of the 5 AIV-positive foxes showed macroscopic brain lesions. Microscopically, we observed mild-to-moderate multifocal lymphohistiocytic encephalitis with predominant perivascular infiltrations in the midbrain of 4 foxes and in the brain stem of 3 foxes. One animal showed vasculitis. Minor lesions were seen in the rhinencephalon and the cerebellum in 2 foxes. Neuronal necrosis was present in the midbrain and rhinencephalon in 1 animal, whereas another animal showed multifocal gliosis in the brain stem and midbrain. Immunohistologic analysis showed influenza A nucleoprotein in morphologically affected and unaffected areas of the brain. This protein was located in the nucleus and perikaryon and cell processes of neurons, as well as in glial cells (Figure 2).
Recent H5N1 virus infections with dramatic losses in sea bird breeding colonies in Europe have proven the deleterious implications of a year-round presence of HPAIV in northern Europe (13). The sustained occurrence of HPAIV outbreaks in wild birds might enhance spillover risks to wild carnivores that prey on infected birds or scavenge on their carcasses. An alimentary route of infection has been proven experimentally (14).
Our phylogenetic analyses confirm that virus strains similar to those circulating in the wild bird population have the potential to be transferred to terrestrial carnivores. Wild birds are suspected to be the most likely animal reservoirs sustaining HPAIV replication in Europe. The role of other animal species, in particular mammalian predators, is still equivocal. Clinically conspicuous cases have been reported throughout Europe in a sporadic fashion, but the true prevalence remains unknown. Our data of a 1-year survey from the federal state Lower Saxony in Germany showed a temporal clustering of cases in red foxes found within the first 3 months of 2023. This period coincided with a peak in HPAIV detections in wild birds in northern Europe (4). Studies from the Netherlands also described positive cases in the winter period of 2021‒2022 (10,11). Conditions in the cold season could favor virus transmission to carnivorous mammals.
Virus variants from foxes in Germany did not form a separate phylogenetic cluster confirming independent infection events. According to several reports, gs/GD-like HPAIV shows a strong neurotropism in mammal species. This finding is true for H5N8 infections in harbor seals (9), as well as for disease outbreaks in terrestrial predators (7,8,10,11). Also, in this study, HPAIV infection of the brain was shown by high viral loads and immunohistochemical analysis. In previous studies, point mutations suspected to increase viral replication in mammals have been frequently described, especially the E627K mutation in the PB2 segment. Ten of 14 HPAIV H5N1-positive wild carnivores detected in the Netherlands carried the mammal-adaptive variant (10,11), and those viruses replicated to higher titers in mammalian cells than in an avian cell line (10). In our study, only 1 of 4 four analyzed cases had the E627K substitution. Those results support previous observations that PB2 627K is not a prerequisite for virus replication in mammalian cells (10).
Little is known about the pathogenesis of HPAIV infection in wild mammal predators. Further virologic and serologic studies are planned and are needed to monitor those potential hosts as indicators for enhanced zoonotic spillover. Recent serologic findings of a clinically silent HPAIV H5N1 infection in a pig herd in Italy suggest that the neurologic cases seen in carnivores in Europe and elsewhere might represent just the tip of an iceberg (15). Widespread HPAIV H5N1 infection in those hosts would provide ample opportunities to further adapt to mammals, which could be associated with increased infection risks for humans.
Dr. Baechlein is a research scientist at the Lower Saxony State Office for Consumer Protection and Food Safety in Braunschweig/Hannover, Germany. Her primary research interest is characterization of zoonotic and animal disease pathogens.
Acknowledgment
We thank the local authorities in Lower Saxony for submitting wild animals and Sabine Baumann, Hannah Habeck, Sina Korn, Bozena Ohlhäuser, Sara Walter, Diana Wessler, and Kristin Trippler for excellent technical assistance with diagnostic samples and virus isolation.
References
- King J, Harder T, Conraths FJ, Beer M, Pohlmann A. The genetics of highly pathogenic avian influenza viruses of subtype H5 in Germany, 2006-2020. Transbound Emerg Dis. 2021;68:1136–50. DOIPubMedGoogle Scholar
- Verhagen JH, Fouchier RAM, Lewis N. Highly pathogenic avian influenza viruses at the wild–domestic bird interface in Europe: future directions for research and surveillance. Viruses. 2021;13:212. DOIPubMedGoogle Scholar
- Pohlmann A, King J, Fusaro A, Zecchin B, Banyard AC, Brown IH, et al. Has epizootic become enzootic? Evidence for a fundamental change in the infection dynamics of highly pathogenic avian influenza in Europe, 2021. MBio. 2022;13:
e0060922 . DOIPubMedGoogle Scholar - Adlhoch C, Fusaro A, Gonzales JL, Kuiken T, Marangon S, Mirinaviciute G, et al.; European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza. Avian influenza overview December 2022 - March 2023. EFSA J. 2023;21:
e07917 .PubMedGoogle Scholar - Puryear W, Sawatzki K, Hill N, Foss A, Stone JJ, Doughty L, et al. Highly pathogenic avian influenza A (H5N1) virus outbreak in New England seals, United States. Emerg Infect Dis. 2023;29:786–91. DOIPubMedGoogle Scholar
- Agüero M, Monne I, Sánchez A, Zecchin B, Fusaro A, Ruano MJ, et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill. 2023;28:
2300001 . DOIPubMedGoogle Scholar - Floyd T, Banyard AC, Lean FZX, Byrne AMP, Fullick E, Whittard E, et al. Encephalitis and death in wild mammals at a rehabilitation center after infection with highly pathogenic avian influenza A (H5N8) virus, United Kingdom. Emerg Infect Dis. 2021;27:2856–63. DOIPubMedGoogle Scholar
- Rijks JM, Hesselink H, Lollinga P, Wesselman R, Prins P, Weesendorp E, et al. Highly pathogenic avian influenza A (H51) virus in wild red foxes, the Netherlands, 2021. Emerg Infect Dis. 2021;27:2960–2. DOIPubMedGoogle Scholar
- Postel A, King J, Kaiser FK, Kennedy J, Lombardo MS, Reineking W, et al. Infections with highly pathogenic avian influenza A virus (HPAIV) H5N8 in harbor seals at the German North Sea coast, 2021. Emerg Microbes Infect. 2022;11:725–9. DOIPubMedGoogle Scholar
- Bordes L, Vreman S, Heutink R, Roose M, Venema S, Pritz-Verschuren SBE, et al. Highly pathogenic avian influenza H5N1 virus infections in wild red foxes (Vulpes vulpes) show neurotropism and adaptive virus mutations. Microbiol Spectr. 2023;11:
e0286722 . DOIPubMedGoogle Scholar - Vreman S, Kik M, Germeraad E, Heutink R, Harders F, Spierenburg M, et al. Zoonotic mutation of highly pathogenic avian influenza H5N1 virus identified in the brain of multiple wild carnivore species. Pathogens. 2023;12:168. DOIPubMedGoogle Scholar
- Pohlmann A, Stejskal O, King J, Bouwhuis S, Packmor F, Ballstaedt E, et al. Mass mortality among colony-breeding seabirds in the German Wadden Sea in 2022 due to distinct genotypes of HPAIV H5N1 clade 2.3.4.4b. J Gen Virol. 2023;104:104. DOIPubMedGoogle Scholar
- Reperant LA, van Amerongen G, van de Bildt MW, Rimmelzwaan GF, Dobson AP, Osterhaus ADME, et al. Highly pathogenic avian influenza virus (H5N1) infection in red foxes fed infected bird carcasses. Emerg Infect Dis. 2008;14:1835–41. DOIPubMedGoogle Scholar
- Rosone F, Bonfante F, Sala MG, Maniero S, Cersini A, Ricci I, et al. Seroconversion of a swine herd in a free-range rural multi-species farm against HPAI H5N1 2. 3. 4. 4b clade virus. Microorganisms. 2023;11:1162. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: October 31, 2023
Table of Contents – Volume 29, Number 12—December 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correspondence correction: Christine Baechlein, Lower Saxony State Office for Consumer Protection and Food Safety, Food and Veterinary Institute Braunschweig/Hannover, Eintrachtweg 17, 30173 Hannover, Germany
Top