Volume 29, Number 2—February 2023
Dispatch
Molecular Detection of Candidatus Orientia chuto in Wildlife, Saudi Arabia
Figure 1
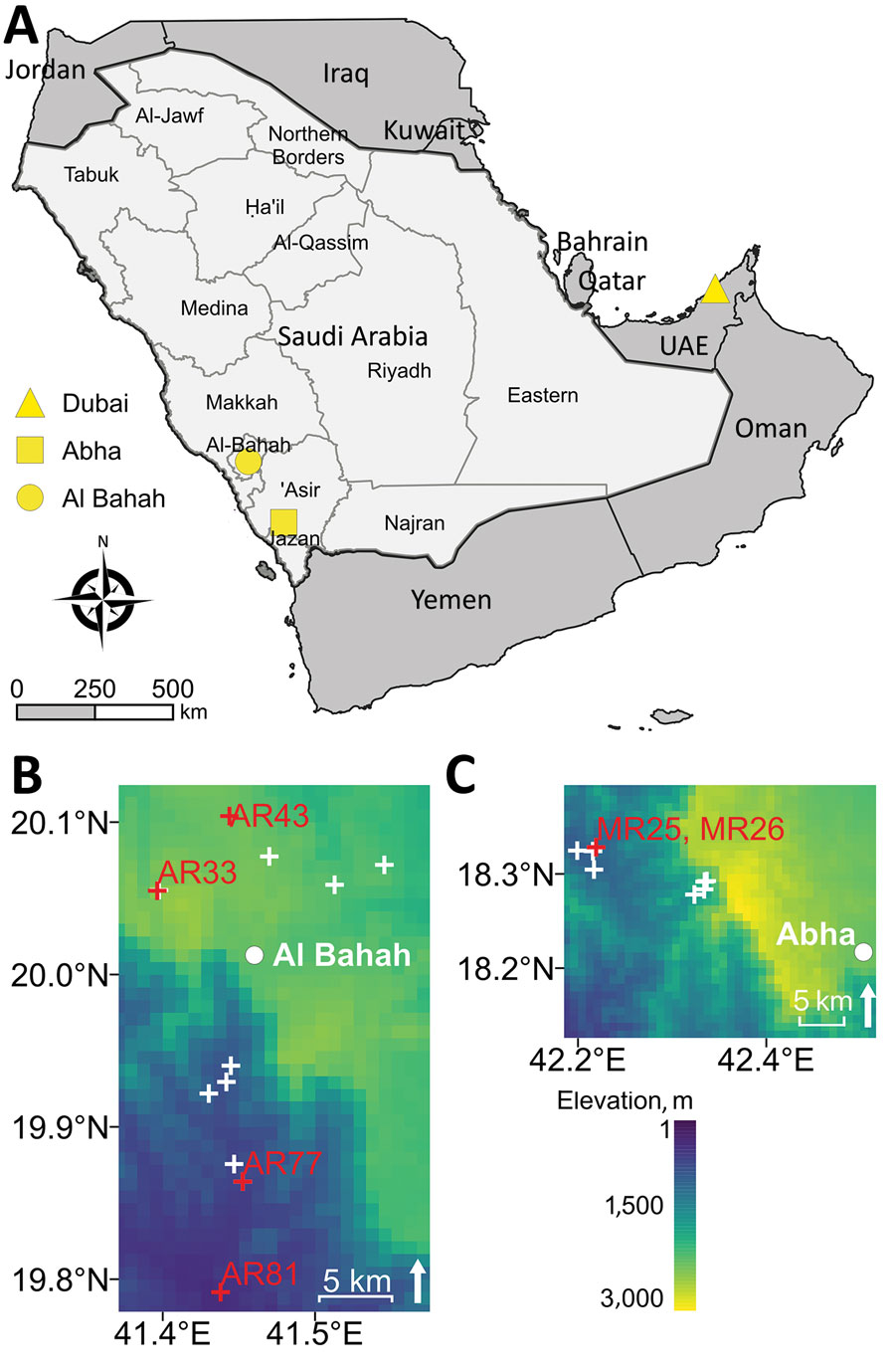
Figure 1. Sampling sites and elevation from which rodents were collected for molecular detection of Candidatus Orientia chuto in wildlife, Saudi Arabia. A) Study region on the Arabian Peninsula, including Dubai (yellow triangle), where a clinical case of scrub typhus caused by Candidatus O. chuto was reported in a previous study (5). Light gray area indicates Saudi Arabia; dark gray area indicates bordering countries on the Arabian Peninsula. Rodents were trapped in the Hijaz Mountains and surrounding towns of Al-Bahah Province (yellow circle indicates Al-Bahah, the capital city) and in the Asir Mountains of Asir Province (yellow square indicates Abha, the capital city). B, C) Heat maps detailing elevation above sea level of trapping locations in Al-Bahah Province (B) and Asir Province (C). Red crosses and sample labels indicate where Orientia-positive rodents were found; white crosses indicate areas in which rodents showed no evidence of infection. UAE, United Arab Emirates.
References
- Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: A systematic review. PLoS Negl Trop Dis. 2017;11:
e0005838 . DOIPubMedGoogle Scholar - McGready R, Prakash JAJ, Benjamin SJ, Watthanaworawit W, Anantatat T, Tanganuchitcharnchai A, et al. Pregnancy outcome in relation to treatment of murine typhus and scrub typhus infection: a fever cohort and a case series analysis. PLoS Negl Trop Dis. 2014;8:
e3327 . DOIPubMedGoogle Scholar - Traub R, Wisseman CL Jr, Jones MR, O’Keefe JJ. The acquisition of Rickettsia tsutsugamushi by chiggers (trombiculid mites) during the feeding process. Ann N Y Acad Sci. 1975;266(1 Pathobiology):91–114.
- Elliott I, Pearson I, Dahal P, Thomas NV, Roberts T, Newton PN. Scrub typhus ecology: a systematic review of Orientia in vectors and hosts. Parasit Vectors. 2019;12:513. DOIPubMedGoogle Scholar
- Izzard L, Fuller A, Blacksell SD, Paris DH, Richards AL, Aukkanit N, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404–9. DOIPubMedGoogle Scholar
- Masakhwe C, Linsuwanon P, Kimita G, Mutai B, Leepitakrat S, Yalwala S, et al. Identification and characterization of Orientia chuto in trombiculid chigger mites collected from wild rodents in Kenya. J Clin Microbiol. 2018;56:e01124–18. DOIPubMedGoogle Scholar
- Abarca K, Martínez-Valdebenito C, Angulo J, Jiang J, Farris CM, Richards AL, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148–56. DOIPubMedGoogle Scholar
- Stekolnikov AA, Al-Ghamdi SQ, Alagaili AN, Makepeace BL. First data on chigger mites (Acariformes: Trombiculidae) of Saudi Arabia, with a description of four new species. Syst Appl Acarol. 2019;24:1937–63. DOIGoogle Scholar
- Chao C-C, Belinskaya T, Zhang Z, Jiang L, Ching W-M. Assessment of a sensitive qPCR assay targeting a multiple-copy gene to detect Orientia tsutsugamushi DNA. Trop Med Infect Dis. 2019;4:113. DOIPubMedGoogle Scholar
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42. DOIPubMedGoogle Scholar
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36(Suppl 2):
W465-9 . DOIPubMedGoogle Scholar - Posada D. jModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–6. DOIPubMedGoogle Scholar
- Cosson JF, Galan M, Bard E, Razzauti M, Bernard M, Morand S, et al. Detection of Orientia sp. DNA in rodents from Asia, West Africa and Europe. Parasit Vectors. 2015;8:172. DOIPubMedGoogle Scholar
- Al-Mekhlafi HM, Madkhali AM, Ghailan KY, Abdulhaq AA, Ghzwani AH, Zain KA, et al. Residual malaria in Jazan region, southwestern Saudi Arabia: the situation, challenges and climatic drivers of autochthonous malaria. Malar J. 2021;20:315. DOIPubMedGoogle Scholar
- Alhaeli A, Bahkali S, Ali A, Househ MS, El-Metwally AA. The epidemiology of Dengue fever in Saudi Arabia: A systematic review. J Infect Public Health. 2016;9:117–24. DOIPubMedGoogle Scholar