Volume 29, Number 4—April 2023
Dispatch
Chikungunya Outbreak in Country with Multiple Vectorborne Diseases, Djibouti, 2019–2020
Cite This Article
Citation for Media
Abstract
During 2019–2020, a chikungunya outbreak occurred in Djibouti City, Djibouti, while dengue virus and malaria parasites were cocirculating. We used blotting paper to detect arbovirus emergence and confirm that it is a robust method for detecting and monitoring arbovirus outbreaks remotely.
Djibouti is a semi-arid country bordered by Eritrea, Somalia, and Ethiopia. In the region, the main vector of chikungunya virus (CHIKV) and dengue virus (DENV) is the Aedes aegypti mosquito. The French Armed Forces are stationed in Djibouti City, where 70% of the country’s population live (total population ≈900,000). Military bases and housing are located in the urban area, and the entire French Defense Community (FDC), including service members, families, and civilian employees, comprise a population of 2,700.
During July–October 2019, a large-scale chikungunya outbreak (41,162 suspected cases, 16 laboratory-confirmed cases, attack rate 12.3%) occurred in Dire Dawa, Ethiopia, 260 km from Djibouti City (Appendix Figure) (1). In a 2010–2011 survey in Djibouti City, although no epidemic has been reported since, 2.6% of the population had serologic evidence of CHIKV infection (2). Given the road, rail, and air connections between the 2 cities and the CHIKV-naive local populations, we estimated the likelihood of a CHIKV outbreak in Djibouti City to be highly probable. Patient management was challenging because dengue fever and malaria are endemic to Djibouti (3).
We describe the comprehensive response implemented by the FDC to these multiple vectorborne diseases and evaluated the use of blood on blotting paper for arboviral diagnosis. With the consent of patients, we collected and anonymized epidemiologic and clinical data for diagnostic purposes. According to French regulations, because this outbreak was considered an immediate threat to public health, ethics approval was not required for this investigation.
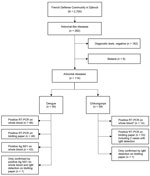
In October 2019, we strengthened epidemiologic surveillance in the FDC to detect CHIKV emergence. We defined a suspected case of arboviral-like disease (ALD) as fever or chills and/or acute arthralgia and/or rash and/or vomiting and diarrhea. Symptomatic patients were encouraged to seek medical care for systematic testing for dengue, chikungunya, and malaria. From each person with ALD signs/symptoms, we collected venous blood, spotted it onto Whatman 3MM blotting paper (Sigma-Aldrich, https://www.sigmaaldrich.com), dried the samples at room temperature, and stored them in a sealed plastic pouch for preservation and transport (4). The National Reference Center for arboviruses in France performed reverse transcription PCR (RT-PCR) and serologic testing for CHIKV and DENV on blotting paper as described elsewhere (5). In January, equipment was set up locally to perform in-house RT-PCR for DENV and CHIKV on whole-blood samples (Figure 1) (6,7). Chikungunya cases were confirmed by positive RT-PCR on whole blood or blotting paper or by detection of CHIKV IgM on blotting paper. Dengue cases were confirmed by a positive DENV RT-PCR on whole blood or blotting paper or a positive nonstructural protein 1 (NS1) antigen rapid diagnostic test (RDT) (Bioline Dengue Duo; Abbott, https://www.abbott.com). We provided care according to the French National Recommendations (8) and World Health Organization guidelines (9). Concurrently, we strengthened the following in the FDC vector-control measures and personal protection: larval source management, long clothing, insect repellents, and long-lasting insecticidal nets.
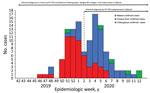
We compared clinical presentations of dengue and chikungunya by using R version 3.5.1 software (The R Project for Statistical Computing, https://www.r-project.org) for statistical analyses. Overall, among the 2,700 persons in the FDC, we included 282 with ALD. Through March 2020, we confirmed 120 cases of vectorborne disease (attack rate 42.6%, 120/282): 58 chikungunya (2.1%, 58/2,700), 56 dengue (2.1%), 6 malaria (5 Plasmodium falciparum and 1 P. vivax), and no co-infections (Figure 2). We also documented 2 concomitant influenza A virus and arbovirus infections. Among patients with vectorborne diseases, 67.5% (81/120) were male, and 73.3% (88/120) were service members. The median age was 34.5 (range 8.3–79.6, interquartile range 27.1–40.0) years, and 92.5% (111/120) of persons sought care within 48 hours of symptom onset (median 1, range 0–7, interquartile range 0–1 days).
We confirmed the first chikungunya case among persons in the FDC in November 2019. The outbreak started in December and lasted 13 weeks. The CHIKV strain belonged to the Indian lineage of the East/Central/South African genotype (10). The dengue outbreak peaked in late January and was linked to DENV-1 with a unique serotype, confirmed for 36/56 dengue cases (Figure 2). CHIKV and DENV co-circulated for 16 weeks. One chikungunya case was diagnosed by CHIKV IgM on blotting paper alone; all others (57/58, 98%) were confirmed by RT-PCR. One dengue case was diagnosed by positive NS1 antigen RDT with positive DENV IgM on blotting paper; all others (55/56, 98%) were confirmed by RT-PCR (Figure 1). The National Reference Center received blotting paper samples for 93.0% (106/114) of the DENV and CHIKV infections and confirmed 97.2% (103/106) of the diagnoses, 93.4% (99/106) by RT-PCR and 3.8% (4/106) by serology (1 DENV and 3 CHIKV). DENV and CHIKV RT-PCR testing were performed both on whole blood and on blotting paper for 44.7% (51/114) (Tables 1, 2). Compared with RT-PCR of whole blood, no RT-PCR of blotting paper produced false-positive results.
Samples from ALD patients were locally tested with NS1 antigen RDT, and 43 (43/120, 36%) results were positive. Results were negative for 13/56 (23%) persons with dengue, all tested within a mean delay of 1.5 (range 0–3) days from symptom onset. Among the 46 with DENV infection confirmed by whole-blood RT-PCR, 36 (78%) had concomitant positive RDT results.
The main ALD sign was fever (90.8%, 109/120). Headaches and digestive disorders were more associated with dengue fever (odds ratio [OR] 7.2, 95% CI 2.3–22.8) than chikungunya (OR 5.9, 95% CI 1.8–19.6) (Appendix Table). Highly predictive of chikungunya were arthralgia of the toe (OR 29.97, 95% CI 3.19–195.61), ankle (OR 18.28, 95% CI 6.14-54.71), finger (OR 12.47, 95% CI 3.93–39.61), and wrist (OR 18.27, 95% CI 5.71–58.52). Secondary infection developed in 4 patients with chikungunya (1 case each of pneumonia, dysentery, herpetic recurrence, and gingivitis with oral candidiasis). Among dengue patients, 4 had hepatic cytolysis (maximum transaminases elevation 12 times the upper limit), and 3 had secondary infections including acute pneumonia, Escherichia coli pyelonephritis, and intestinal amoebiasis. No patient met criteria for having severe dengue. No ALD patient required intensive care. All malaria patients recovered after a 3-day course of artenimol/piperaquine and secondary treatment with primaquine treatment for the patient with P. vivax infection. Treatment of arboviral disease relied essentially on analgesics, antihistamines, and hydration. The prescription of nonsteroidal anti-inflammatory drugs, aspirin, or corticosteroids was formally contraindicated during the first days of any infection. For patients with confirmed chikungunya, we carefully assessed the benefit-risk balance of introducing nonsteroidal anti-inflammatory drugs.
Despite recent improvement in diagnostic tools, chikungunya outbreaks in Africa are probably underreported (11). During 2019–2020, a large-scale chikungunya outbreak occurred in Djibouti City (12). However, because of lack of diagnostic tests and dedicated reporting, no data are available to estimate its extent. The chikungunya outbreak remained limited (attack rate 2.1%) in the FDC but was followed by a dengue outbreak. We found that clinical features are helpful but not sufficient to discriminate between chikungunya and dengue (13,14). Biological confirmation remains necessary for determining appropriate care. The use of blood samples on blotting paper has been described as a field method for detecting arboviruses (4,5), routinely used in the French Armed Forces when deployed in Africa (15). In this study, we used blood samples on blotting paper to detect emergence of CHIKV and monitor the course of the outbreaks. Blotting paper provided a robust method for blood sampling and transport to a reference laboratory, making it possible to confirm 90% of the arboviral diagnoses. We recommend blotting paper as a field tool to detect and monitor arboviral epidemics remotely.
Dr. Javelle is a military physician and infectious diseases specialist at the Laveran Military Teaching Hospital and conducts research at the French Armed Forces Biomedical Research Institute and at the University Hospital Institute Méditerranée-Infection, in Marseille, France. She has clinical and scientific experience in vector-borne diseases and travel medicine.
Acknowledgment
We thank Patrick Gravier, David Fery, Pierre Blanco de Torre, Christophe Bodelot, Sandrine Duron, Olivier Cabre, Olivier Merle, Madjid Mokrane, Christelle Tong, Marion Fossier, Diane Houssin, and Jérôme Desplans for their help with this study.
References
- Geleta D, Tesfaye N, Ayigegn H, Waldetensai A, Gemechu F, Amare H. Epidemiological description of chikungunya virus outbreak in Dire Dawa Administrative City, western Ethiopia, 2019. Int J Clin Exp Med Sci. 2020;6:41. DOIGoogle Scholar
- Andayi F, Charrel RN, Kieffer A, Richet H, Pastorino B, Leparc-Goffart I, et al. A sero-epidemiological study of arboviral fevers in Djibouti, Horn of Africa. PLoS Negl Trop Dis. 2014;8:
e3299 . DOIPubMedGoogle Scholar - de Santi VP, Khaireh BA, Chiniard T, Pradines B, Taudon N, Larréché S, et al. Role of Anopheles stephensi mosquitoes in malaria outbreak, Djibouti, 2019. Emerg Infect Dis. 2021;27:1697–700. DOIPubMedGoogle Scholar
- Andriamandimby SF, Heraud JM, Randrianasolo L, Rafisandratantsoa JT, Andriamamonjy S, Richard V. Dried-blood spots: a cost-effective field method for the detection of Chikungunya virus circulation in remote areas. PLoS Negl Trop Dis. 2013;7:
e2339 . DOIPubMedGoogle Scholar - Matheus S, Huc P, Labeau B, Bremand L, Enfissi A, Merle O, et al. The use of serum spotted onto filter paper for diagnosing and monitoring Chikungunya virus infection. J Clin Virol. 2015;71:89–92. DOIPubMedGoogle Scholar
- Pastorino B, Bessaud M, Grandadam M, Murri S, Tolou HJ, Peyrefitte CN. Development of a TaqMan RT-PCR assay without RNA extraction step for the detection and quantification of African Chikungunya viruses. J Virol Methods. 2005;124:65–71. DOIPubMedGoogle Scholar
- Leparc-Goffart I, Baragatti M, Temmam S, Tuiskunen A, Moureau G, Charrel R, et al. Development and validation of real-time one-step reverse transcription-PCR for the detection and typing of dengue viruses. J Clin Virol. 2009;45:61–6. DOIPubMedGoogle Scholar
- Simon F, Javelle E, Cabie A, Bouquillard E, Troisgros O, Gentile G, et al.; Société de pathologie infectieuse de langue francaise. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med Mal Infect. 2015;45:243–63. DOIPubMedGoogle Scholar
- World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control: new edition. Geneva: The Organization; 2009 [cited 2022 Nov 5]. https://apps.who.int/iris/handle/10665/44188
- Fourié T, Dia A, Savreux Q, Pommier de Santi V, de Lamballerie X, Leparc-Goffart I, et al. Emergence of Indian lineage of ECSA chikungunya virus in Djibouti, 2019. Int J Infect Dis. 2021;108:198–201. DOIPubMedGoogle Scholar
- Bettis AA, L’Azou Jackson M, Yoon IK, Breugelmans JG, Goios A, Gubler DJ, et al. The global epidemiology of chikungunya from 1999 to 2020: A systematic literature review to inform the development and introduction of vaccines. PLoS Negl Trop Dis. 2022;16:
e0010069 . DOIPubMedGoogle Scholar - News Desk. Chikungunya epidemic in Djibouti: “Unprecedented”, according to media report. Outbreak News Today. 2020 Jan 18. [cited 2022 Jan 25]. http://outbreaknewstoday.com/chikungunya-epidemic-in-djibouti-unprecedented-according-to-media-report-32763
- Bonifay T, Vesin G, Bidaud B, Bonnefoy C, Dueymes M, Nacher M, et al. Clinical characteristics and predictive score of dengue vs. chikungunya virus infections. Med Mal Infect. 2019;49:250–6. DOIPubMedGoogle Scholar
- Thiberville S-D, Boisson V, Gaudart J, Simon F, Flahault A, de Lamballerie X. Chikungunya fever: a clinical and virological investigation of outpatients on Reunion Island, South-West Indian Ocean. PLoS Negl Trop Dis. 2013;7:
e2004 . DOIPubMedGoogle Scholar - Tong C, Javelle E, Grard G, Dia A, Lacrosse C, Fourié T, et al. Tracking Rift Valley fever: From Mali to Europe and other countries, 2016. Euro Surveill. 2019;24:
1800213 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: March 18, 2023
Table of Contents – Volume 29, Number 4—April 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Vincent Pommier de Santi, Centre d’épidémiologie et de santé publique des armées, GSBdD Marseille Aubagne—CESPA—BP 40029, Marseille 13568, France
Top