Volume 29, Number 6—June 2023
Online Report
One Health Approach for Reporting Veterinary Carbapenem-Resistant Enterobacterales and Other Bacteria of Public Health Concern
Cite This Article
Citation for Media
Abstract
A carbapenem-resistant Enterobacterales outbreak at a veterinary teaching hospital in the United States increased urgency for improved communication among diagnostic laboratories, public health authorities, veterinarians, and pet owners. Kansas State University, University of Missouri, Kansas Department of Health and Environment, and Veterinary Laboratory Investigation and Response Network created a surveillance, storage, and reporting protocol for veterinary antimicrobial-resistant bacteria; determined frequency of those bacteria in companion animals during 2018–2021; and created educational flyers for veterinarians and pet owners. We recommend a One Health strategy to create efficient surveillance programs to identify and report antimicrobial-resistant bacteria and educate veterinarians and pet owners about transmission risks.
Companion animals share living environments with their owners, creating opportunities for sharing bacteria (1–9). Bacteria sharing can have substantial public health consequences because of emerging antimicrobial-resistant (AMR) bacteria, such as carbapenem-resistant Enterobacterales (CRE), carbapenem-resistant Pseudomonas aeruginosa (CRPA), and methicillin-resistant Staphylococcus, which can cause severe human infections and limit options for antimicrobial therapy. The Centers for Disease Control and Prevention (CDC) considers CRE to be an urgent threat and both CRPA and methicillin-resistant Staphylococcus to be serious human health threats (10); carbapenemase-producing CRE are of highest clinical concern and warrant a public health response (11).
CRE bacteria have been isolated from pets, but the true prevalence is unknown (12–14). However, in 2020, the University of Pennsylvania School of Veterinary Medicine reported a cluster of canine and feline CRE cases (15); those cases brought awareness and urgency to One Health professionals to create veterinary laboratory and hospital protocols for CRE reporting and response to improve patient management and minimize transmission and public health effects.
Many states do not require reporting of specific AMR bacteria isolated from veterinary patients, yet the emergence of CRE and other AMR organisms in veterinary medicine has accelerated discussion of whether reporting should be required. Nationally, the Veterinary Laboratory Investigation and Response Network (Vet-LIRN) within the US Food and Drug Administration’s Center for Veterinary Medicine collaborates with laboratories in North America to perform veterinary AMR bacteria monitoring and can assist with further classification of mechanisms and outbreak investigations (16). Establishing a best practice protocol for internal laboratory tracking of AMR isolates and logistical case reporting to state public health authorities will enable efficient epidemiologic tracing and outbreak investigations, if needed. Furthermore, implementing a targeted response with educational material for veterinarians and pet owners will improve patient care and public health when AMR organisms are isolated from pets.
The goals of this study were to create a protocol to routinely surveil, store, and report CRE isolates and other bacteria of public health concern to state and national public health authorities; determine the prevalence of CRE, CRPA, and methicillin-resistant Staphylococcus in companion animals reported by the Kansas State Veterinary Diagnostic Laboratory (KSVDL) and University of Missouri Veterinary Medical Diagnostic Laboratory (MU-VMDL) during 2018–2021; and create educational flyers for veterinarians and pet owners that can be attached to bacteriology reports when CRE, CRPA, or methicillin-resistant Staphylococcus are found in companion animals, providing immediate access information, improved responses, and minimization of public health effects. A One Health approach was used in collaboration with veterinary and human healthcare professionals at local, state, and national agencies to create effective protocols and educational flyers that recognize and respond to public health concerns and reduce the risk of disease in animals and humans. We report the generation of protocols and flyers and the unique challenges associated with implementation encountered by KSVDL and MU-VMDL.
We reviewed and streamlined the current KSVDL methods for storing AMR isolates. We created an efficient standard operating procedure to be applied within the KSVDL and MU-VMDL and made available at other diagnostic laboratories.
We reviewed regulations for reporting AMR organisms in Kansas and Missouri and created a prototype reporting form to enable sharing of pertinent data among the Kansas Department of Health and Environment (KDHE), Missouri Department of Health and Senior Services (MDHSS), and Vet-LIRN. We established optimal methods for identifying applicable isolates and routes of electronic report submission to KDHE and MDHSS.
We searched KSVDL and MU-VMDL records for all CRE, CRPA, and methicillin-resistant Staphylococcus isolates collected from any animal species during 2018–2021. We also collected total numbers of cultures with Escherichia coli or Klebsiella, Proteus, Pseudomonas, or Staphylococcus spp. growth for each host species. We summarized all data and performed descriptive statistical analyses. We did not use human participants or animals in our study; thus, ethical approval was not required.
We created 12 flyers to provide targeted education and improve veterinary response when AMR isolates were identified. We created separate flyers for CRE, CRPA, and methicillin-resistant Staphylococcus for distribution to small animal veterinarians, dog and cat owners, equid veterinarians, and horse owners. Flyer content for veterinarians provided information on bacterial transmission, infection prevention, patient management strategies, and public health considerations. Pet owner flyers were written using lay terms and included information about organisms and sources, guided owners to closely follow veterinarian recommendations, discussed transmission risks to humans, and provided precautions for the home, including cleaning suggestions. All flyers provided additional resource information. Flyer content was initially reviewed by microbiologists, veterinarians, infectious disease specialists, and epidemiologists at KDHE and Vet-LIRN, then reviewed for content, style, and distribution logistics by 3 regional general practice veterinarians. Flyers were reviewed by healthcare communication experts and a graphics designer, who edited and finalized the content.
Protocol for Isolating and Storing CRE, CRPA, and Methicillin-Resistant Staphylococcus
KSVDL and MU-VMDL are accredited by the American Association of Veterinary Laboratory Diagnosticians and follow standard methods for bacteria isolation, identification, and antimicrobial susceptibility testing. Both laboratories use matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Biotyper; Bruker Corp., https://www.bruker.com) to identify bacteria. KSVDL reports the S. intermedius group for isolates that includes S. pseudintermedius, S. intermedius, and S. delphini. Because S. pseudintermedius is known to be the primary canine and feline pathogen in this group, S. intermedius group isolates reported by the KSVDL are interpreted by practicing veterinarians and referred to throughout this report as S. pseudintermedius (17,18). Both laboratories perform antimicrobial susceptibility tests by using Sensititre broth microwell dilution testing and provide MICs and interpretations in accordance with Clinical Laboratory Standards Institute standards (19).
Both laboratories have traditionally used imipenem as a representative carbapenem for bacteria susceptibility testing. Human breakpoints are used because no veterinary imipenem breakpoints are available (19). Imipenem breakpoints for Enterobacterales are <1 µg/mL for susceptible, 2 µg/mL for intermediate, and >4 µg/mL for resistant bacteria. For Pseudomonas spp., breakpoints are <2 µg/mL for susceptible, 4 µg/mL for intermediate, and >8 µg/mL for resistant bacteria. Further carbapenem testing is required to characterize Proteus, Morganella, and Providencia spp. as CRE because of intrinsically elevated MICs for imipenem (11). As of January 1, 2021, MU-VMDL began using both imipenem and meropenem as representative carbapenems for susceptibility testing; organisms showing phenotypic resistance to imipenem are tested further by using a meropenem Etest (bioMérieux, https://www.biomerieux.com). Meropenem breakpoints for Enterobacterales are <1 µg/mL for susceptible, 2 µg/mL for intermediate, and >4 µg/mL for resistant bacteria and, for Pseudomonas aeruginosa, <2 µg/mL for susceptible, 4 µg/mL for intermediate, and >8 µg/mL for resistant bacteria. No veterinary meropenem breakpoints are available; thus, human meropenem breakpoints are used (20). In 2021, KSVDL began sending imipenem-resistant isolates to MU-VMDL for meropenem testing. We excluded Proteus, Morganella, and Providencia spp. identified before 2021 that did not undergo meropenem testing from this study. Carbapenemase production was not analyzed at either laboratory.
KSVDL and MU-VMDL use oxacillin as their representative penicillinase-resistant β-lactam antimicrobial. Oxacillin breakpoints are <2 µg/mL for susceptible and >4 µg/mL for resistant S. aureus and <0.25 µg/mL for susceptible and >0.5 µg/mL for resistant S. pseudintermedius (19). In accordance with CDC guidelines, S. aureus isolates showing phenotypic oxacillin resistance are tested further by using cefoxitin disks (Hardy Diagnostics, https://www.hardydiagnostics.com) and the Kirby-Bauer disk diffusion method. According to CDC guidelines, cefoxitin disk diffusion testing uses breakpoints (for zones of inhibition) of >22 mm for susceptible and <21 mm for resistant S. aureus. Coagulase-positive non–S. aureus staphylococci do not require cefoxitin disk diffusion testing to be classified as methicillin resistant (21).
In both laboratories, new CRE, CRPA, and methicillin-resistant Staphylococcus isolates from any animal host species are recultured weekly and stored in cryogenic storage vials at −80°C for further testing. Cryogenic tubes are labeled with unique identifying numbers, animal species, specimen type, genus and species, date, and initials of the microbiologist who froze the isolate. After the tubes are labeled, a sterile loop is used to pick colonies, which are then immersed in CryoSaver, Brucella Broth with Glycerol (10%) and Beads solution (Hardy Diagnostics) in cryogenic tubes. The tubes are vortexed and left at room temperature for 15 min. A sterile plastic pipette is used to remove the liquid, and tubes are stored at −80°C.
Recording and Reporting CRE, CRPA, and Methicillin-Resistant Staphylococcus Data
We created a prototype reporting spreadsheet for animal isolate submissions that includes the submitting laboratory, date culture was finalized, animal species, specimen type and source (e.g., swab from ear), testing method, species, antimicrobials tested, MIC, and MIC interpretation (Appendix). We maintained confidentiality by excluding names of veterinarians, clinics, pet owners, pets, and contact information.
On the first day of each month, a report of CRE, CRPA, and methicillin-resistant Staphylococcus isolated by KSVDL was electronically generated by using an electronic medical record system (VetView version 2.0.18, https://www.vetview.org) and no-code workflow software (Decisions 6.7, https://www.decisions.com) to search the KSVDL database; search criteria was imipenem-resistant organisms isolated from any animal species. At MU-VMDL, the Sensititre SWIN software (Thermo Fisher Scientific, https://www.thermofisher.com) database was updated manually by using custom reporting categories each time a methicillin-resistant Staphylococcus or CRE organism was detected. The SWIN database was searched monthly for both methicillin-resistant Staphylococcus and CRE; data describing isolates were exported as a .csv file (comma-separate values), and duplicate entries were removed. Both laboratories maintain a comprehensive internal spreadsheet of clearly labeled stored isolates that can be added onto if further testing is performed, such as additional susceptibility tests or whole-genome sequencing.
In Kansas, human diagnostic laboratories are required to report human isolates of carbapenem-resistant organisms and vancomycin-intermediate/resistant S. aureus to KDHE within 24 hours and send a bacterial isolate, clinical specimen, or nucleic acid from any carbapenem-resistant organism (22). In Missouri, human isolates of vancomycin-intermediate/resistant S. aureus are reportable within 24 hours, and nosocomial MRSA are reportable quarterly (23). Carbapenem-resistant Enterobacter spp., E. coli, and Klebsiella spp. from human hosts are reportable quarterly as aggregates in Missouri, but carbapenemase-producing CRE are reportable immediately (24). In Kansas and Missouri, reporting is not required if those AMR organisms are isolated from veterinary patients (25,26).
Before sharing monthly surveillance reports with public health authorities outside the KSVDL and MU-VMDL, accession numbers were removed to maintain confidentiality. Then, the KSVDL spreadsheet was emailed to the AMR bacteria epidemiology team at KDHE, and the KSVDL and MU-VMDL spreadsheets were emailed to Vet-LIRN. During 2018–2021, MDHSS did not accept reports of animal-derived CRE, CRPA, or methicillin-resistant Staphylococcus, so direct reporting to the state was not pursued. We implemented the designed protocol for storing and reporting CRE, CRPA, and methicillin-resistant Staphylococcus at the KSVDL and MU-VMDL in January 2021 and have continued using the protocol since then.
Prevalence of AMR Organisms
Bacteria classified as CRE were sporadically isolated from companion animals during 2018–2021 (Appendix). Carbapenem-resistant E. coli was isolated from 2 canids by KSVDL (1 ear swab sample, 1 abscess sample) and from 6 animals by MU-VMDL (2 canine abdominal swab samples, 1 canine ear swab sample, 1 canine blood sample, 1 equine peritoneal fluid sample, and 1 equine wound sample). Carbapenem-resistant E. coli was found in 0.2% (2/1288) of E. coli isolates from canids at KSVDL, 0.2% (4/1705) of isolates from canines at MU-VMDL, and 0.6% (2/331) of isolates from equids at MU-VMDL. Carbapenem-resistant K. pneumoniae was isolated from 3 canids by KSVDL (2 urine samples, 1 wound sample) and from 2 canids by MU-VMDL (1 urine sample, 1 ear swab sample). Carbapenem-resistant K. pneumoniae was found in 1.5% (3/201) of all Klebsiella spp. isolates from canids at KSVDL; carbapenem-resistant Klebsiella spp. were found in 1% (4/409) of all Klebsiella spp. isolated at MU-VMDL.
In 2021, imipenem-resistant Proteus spp. were identified in 44.4% (55/124) of all Proteus spp. isolates from canids, 50% (1/2) of all Proteus spp. isolates from felids, and 50% (1/2) of all Proteus spp. isolates from equids at MU-VMDL as well as in 3% (4/131) of all Proteus spp. isolates from canids and 29% (2/7) of Proteus spp. isolates from felids at KSVDL. All 57 Proteus spp. isolates from MU-VMDL were confirmed as meropenem-susceptible bacteria. Four imipenem-resistant Proteus spp. isolates from KSVDL (3 canine, 1 feline) were sent to MU-VMDL for meropenem testing, and all 4 were meropenem-susceptible bacteria.
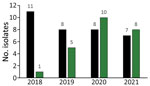
Carbapenem-resistant Pseudomonas spp. were isolated almost exclusively from dogs (Figure 1) but also from 2 cats and 1 horse. The numbers of CRPA isolates/total Pseudomonas spp. isolates from dogs were 11/159 (6.9%) in 2018, 8/150 (5.3%) in 2019, 8/146 (5.5%) in 2020, and 7/178 (3.9%) in 2021 at KSVDL. At MU-VMDL, CRPA isolates were identified in 1/151 (0.07%) dogs in 2018, 5/169 (3.0%) in 2019, 10/166 (6.0%) in 2020, and 8/163 (4.9%) in 2021. CRPA isolates from canids were collected from ear swab samples (n = 32), skin and wounds (n = 12), urogenital samples (n = 9), respiratory samples (n = 3), and unspecified sites (n = 2).
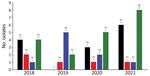
MRSA and methicillin-resistant S. pseudintermedius were isolated by KSVDL and MU-VMDL during 2018–2021 from both dogs and cats (Figures 2, 3). The most common sources of infection were skin (including abscesses, incisions, pyoderma, and wounds) in 34/63 dogs and 9/22 cats, urine (4/63 dogs, 6/22 cats), nose (6/63 dogs, 2/22 cats), ear (3/63 dogs, 3/22 cats), bones or joints (4/63 dogs, 0/22 cats), and the oral cavity (0/63 dogs, 1/22 cats); location was not reported for 15 dogs and 0 cats. Of 334 Staphylococcus spp. isolates from equids at MU-VMDL during 2018–2021, 5 were MRSA (1.5%) and 1 was methicillin-resistant S. pseudintermedius (0.3%). MRSA infections in equids were found in 3 wounds, 1 umbilicus sample, and 1 nasal sample; the sole equine methicillin-resistant S. pseudintermedius isolate was from an abscess. MRSA and methicillin-resistant S. pseudintermedius were not isolated from equine samples at KSVDL during 2018–2021.
To directly communicate the public health importance of AMR isolation and help guide an optimal response, when a CRE, CRPA, or methicillin-resistant Staphylococcus spp. was isolated from a dog, cat, or horse by KSVDL or MU-VMDL, the corresponding veterinarian and pet owner flyers were digitally attached as .pdf (portable document format) files to the final bacteriology report sent to the submitting veterinarian. Those flyers could be downloaded, printed, and shared among veterinary staff and pet owners. The flyers were also posted online and available for download by the public (27–29). To attach flyers, KSVDL used the VetView electronic record system and Decisions 6.7 software, which can be programmed with search criteria, such as imipenem-resistant, E. coli, and dog; for all newly finalized bacteriology reports, flyers were attached automatically to those reports that fit the criteria. At MU-VMDL, VetView was used routinely; however, Decisions software was not available because of cost. Thus, isolates of interest were identified, and flyers were individually attached to each relevant bacteriology report by a microbiologist either digitally for email or manually for fax transmission.
CRE were only sporadically isolated at KSVDL and MU-VMDL during 2018–2021. However, in February 2022, a dog recently imported from Iran to the midwestern United States was confirmed to have New Delhi metallo-β-lactamase-5–producing carbapenem-resistant E. coli, and an additional 18 dogs in the same facility had confirmed CRE in rectal swab samples (30). Those cases reinforce that CRE are an urgent One Health threat. Carbapenem-resistant E. coli isolates from this study were not characterized further to identify carbapenemase production because they were isolated before storage protocols were in place. However, newly isolated CRE from KSVDL and UM-VMDL are now submitted to Vet-LIRN for whole-genome sequencing, when funding is available, to identify resistance genes (31,32). Although CRE prevalence from other US veterinary diagnostic laboratories is unknown and might differ from KSVDL and UM-VMDL, ongoing surveillance and educational efforts are recommended to guide veterinarians and protect pet owners.
MRSA was isolated infrequently from dogs and cats at both university laboratories; methicillin-resistant S. pseudintermedius was observed more frequently at MU-VMDL, likely because the laboratory receives a high case load of samples from 3 specialty dermatology practices. Both MRSA and methicillin-resistant S. pseudintermedius can cause infections of the skin, ears, surgical sites, and urinary tracts of dogs and cats but can also colonize pets without causing active infection. Furthermore, those bacteria are often resistant to many antimicrobial drugs, making treatment challenging (33). Methicillin-resistant Staphylococcus transmission is possible among companion animals, humans, and environmental reservoirs (34). Persons exposed to methicillin-resistant S. pseudintermedius tend to have rapid elimination without becoming clinically ill; however, immunocompromised persons can sometimes develop severe disease (4,35,36). MRSA is more often implicated in the transmission from humans to dogs, although transmission can occur in both directions (6,37). Clonal MRSA originating from humans has also been transmitted to horses, causing nosocomial equine infections (2), which places the horse, owner, rider, and veterinary personnel at risk for infection. Through surveillance and targeted educational flyers, One Health teams consisting of public health authorities, diagnostic laboratories, and veterinary staff can work together to recognize methicillin-resistant Staphylococcus and take actions to minimize further transmission at veterinary clinics and educate pet owners on minimizing transmission risk in the home and barn.
It is critical to use consistent definitions for AMR bacteria to enable clear communication and prevalence comparisons. Using imipenem as a representative carbapenem for susceptibility testing is a limitation at some veterinary diagnostic laboratories, including KSVDL; imipenem-resistant Proteus spp. isolated at KSVDL cannot be characterized as CRE unless further testing is performed (38). Laboratories using only imipenem should explore adding more in-house testing opportunities for Proteus, Morganella, and Providencia spp. or consider sending samples to laboratories with those testing capabilities. Carbapenems are rarely prescribed to companion animals and should be reserved for confirmed resistant infections in consultation with an infectious disease specialist, clinical veterinary pharmacologist, or microbiologist (39). Because clinical veterinarians might not have knowledge of carbapenem testing differences between Enterobacterales species, experts should provide advice and recommendations for additional susceptibility testing, when necessary, to obtain the most accurate susceptibility profile and determine optimal treatment strategies.
Financial resources are an additional challenge encountered by public health agencies monitoring AMR bacteria. Whereas most monitoring is passive and observational, preserving isolates and performing additional AMR bacteria susceptibility testing can be costly. In some cases, the pet owner will pay for additional testing to determine optimal therapy, but testing might also be requested for the sole purpose of improved understanding of regional AMR bacteria incidence. Additional funds can come from diagnostic laboratory or university administration, grant funds, or state public health funds. Dedication of resources (time, personnel, money) is vital for a successful AMR bacteria monitoring program.
Efficiently creating monthly reports of AMR veterinary isolates depends on having an automated electronic laboratory management system and knowledgeable information technology staff available to assist with setup, search parameters, and distribution of reports. At KSVDL, VetView and Decisions software enables creation of monthly excel spreadsheets that have targeted information for AMR bacteria and can be emailed to our research team automatically. However, not all electronic records systems are conducive to creating reports efficiently, and setup might be challenging, expensive, or require manual data collection. MU-VMDL used VetView as their primary record system, but they did not have the Decisions software available because of the additional cost; thus, identifying isolates and creating reports were more time-consuming.
When beginning a veterinary AMR bacteria surveillance and reporting program, who has access to AMR reports and what the intended uses will be should be clarified before reports are provided. Thus far, reports have been used for surveillance and regional prevalence determination of veterinary bacteria isolates and have created an opportunity for One Health discussions with state (KDHE) and federal (Vet-LIRN) public health teams about isolates of concern and additional testing needs. Furthermore, surveillance has enabled communication with veterinarians about those isolates through targeted educational flyers that can improve public health responses.
Collaborations between veterinary diagnostic laboratories and state public health authorities vary widely among US states. Kansas has an active state antimicrobial resistance program and an advisory group composed of statewide One Health professionals (40). Kansas State University veterinarians have an excellent working relationship with the state public health veterinarian and KDHE colleagues, and KDHE stays abreast of veterinary AMR isolates and collaborates on One Health research. In contrast, Missouri has been without a state public health veterinarian for several years, and MDHSS has not been able to invest in veterinary AMR surveillance. For diagnostic laboratories in states without a state public health veterinarian or those that do not have routine collaborative interaction with their state public health authorities, setting up a reporting system for AMR bacteria might be more challenging or less well received.
The primary limitation of our study is that the reach and effects of the educational flyers for veterinarians or pet owners were not measured. A future study could determine the basic level of knowledge of veterinarians regarding AMR organisms and measure knowledge learned or retained from flyers received for patient-specific situations. In addition, future studies could monitor rates of distribution of flyers to pet owners by veterinarians and determine the extent of public health knowledge and AMR transmission risk mitigation by pet owners.
In conclusion, we recommend a One Health strategy for creating an efficient surveillance program to identify and report regional bacterial isolates of concern and educating veterinarians and pet owners about transmission risks. The logistics of establishing such a program come with challenges that are unique to each laboratory. However, the benefits of increased awareness of isolate prevalence over time and having a specific plan for addressing positive cases will enable coordinated efforts that minimize effects of AMR organisms on veterinary patients and public health.
Dr. KuKanich is a professor of small animal veterinary internal medicine and public health at Kansas State University College of Veterinary Medicine. Her research interests focus on infectious and zoonotic diseases and antimicrobial stewardship.
Acknowledgments
We thank Justin Cermak for technical support with electronic record systems; Susie Larson and Gina Scott for assistance with the flyers and figures; Stephanie Lindemann for collaboration and assistance with reporting to the Kansas Department of Health and Environment; Paige Anderson, Molly Brobst, Olgica Ceric, Stephen Cole, Gonzalo Erdozain, Sarah Peloquin, and Kellie Wark for review of flyers; and Olgica Ceric, Sarah Peloquin, and Greg Tyson for collaboration and assistance with reporting to the US Food and Drug Administration Veterinary Laboratory Investigation and Response Network.
This study was funded by the US Food and Drug Administration Veterinary Laboratory Investigation and Response Network (grant no. U18FD006990).
References
- Belas A, Menezes J, Gama LT, Pomba C. Sharing of clinically important antimicrobial resistance genes by companion animals and their human household members. Microb Drug Resist. 2020;26:1174–85. DOIPubMedGoogle Scholar
- Cuny C, Witte W. MRSA in equine hospitals and its significance for infections in humans. Vet Microbiol. 2017;200:59–64. DOIPubMedGoogle Scholar
- Fernandes MR, Sellera FP, Moura Q, Carvalho MPN, Rosato PN, Cerdeira L, et al. Zooanthroponotic transmission of drug-resistant Pseudomonas aeruginosa, Brazil. Emerg Infect Dis. 2018;24:1160–2. DOIPubMedGoogle Scholar
- Frank LA, Kania SA, Kirzeder EM, Eberlein LC, Bemis DA. Risk of colonization or gene transfer to owners of dogs with meticillin-resistant Staphylococcus pseudintermedius. Vet Dermatol. 2009;20:496–501. DOIPubMedGoogle Scholar
- Grönthal T, Österblad M, Eklund M, Jalava J, Nykäsenoja S, Pekkanen K, et al. Sharing more than friendship - transmission of NDM-5 ST167 and CTX-M-9 ST69 Escherichia coli between dogs and humans in a family, Finland, 2015. Euro Surveill. 2018;23:
1700497 . DOIPubMedGoogle Scholar - Lefebvre SL, Reid-Smith RJ, Waltner-Toews D, Weese JS. Incidence of acquisition of methicillin-resistant Staphylococcus aureus, Clostridium difficile, and other health-care-associated pathogens by dogs that participate in animal-assisted interventions. J Am Vet Med Assoc. 2009;234:1404–17. DOIPubMedGoogle Scholar
- Morris DO, Davis MF, Palmeiro BS, O’Shea K, Rankin SC. Molecular and epidemiological characterization of canine Pseudomonas otitis using a prospective case-control study design. Vet Dermatol. 2017;28:118–e25. DOIPubMedGoogle Scholar
- Pomba C, Rantala M, Greko C, Baptiste KE, Catry B, van Duijkeren E, et al. Public health risk of antimicrobial resistance transfer from companion animals. J Antimicrob Chemother. 2017;72:957–68.PubMedGoogle Scholar
- Stenske KA, Bemis DA, Gillespie BE, D’Souza DH, Oliver SP, Draughon FA, et al. Comparison of clonal relatedness and antimicrobial susceptibility of fecal Escherichia coli from healthy dogs and their owners. Am J Vet Res. 2009;70:1108–16. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019 [cited 2023 Mar 2]. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf
- Centers for Disease Control and Prevention. Healthcare-associated infections (HAIs). CRE technical information [cited 2023 Mar 2]. https://www.cdc.gov/hai/organisms/cre/technical-info.html#Definition
- González-Torralba A, Oteo J, Asenjo A, Bautista V, Fuentes E, Alós JI. Survey of carbapenemase-producing Enterobacteriaceae in companion dogs in Madrid, Spain. Antimicrob Agents Chemother. 2016;60:2499–501. DOIPubMedGoogle Scholar
- Köck R, Daniels-Haardt I, Becker K, Mellmann A, Friedrich AW, Mevius D, et al. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: a systematic review. Clin Microbiol Infect. 2018;24:1241–50. DOIPubMedGoogle Scholar
- Yousfi M, Touati A, Mairi A, Brasme L, Gharout-Sait A, Guillard T, et al. Emergence of carbapenemase-producing Escherichia coli isolated from companion animals in Algeria. Microb Drug Resist. 2016;22:342–6. DOIPubMedGoogle Scholar
- Cole SD, Peak L, Tyson GH, Reimschuessel R, Ceric O, Rankin SC. New Delhi metallo-β-lactamase-5–producing Escherichia coli in companion animals, United States. Emerg Infect Dis. 2020;26:381–3. DOIPubMedGoogle Scholar
- Ceric O, Tyson GH, Goodman LB, Mitchell PK, Zhang Y, Prarat M, et al. Enhancing the one health initiative by using whole genome sequencing to monitor antimicrobial resistance of animal pathogens: Vet-LIRN collaborative project with veterinary diagnostic laboratories in United States and Canada. BMC Vet Res. 2019;15:130. DOIPubMedGoogle Scholar
- Fitzgerald JR. The Staphylococcus intermedius group of bacterial pathogens: species re-classification, pathogenesis and the emergence of meticillin resistance. Vet Dermatol. 2009;20:490–5. DOIPubMedGoogle Scholar
- Sasaki T, Kikuchi K, Tanaka Y, Takahashi N, Kamata S, Hiramatsu K. Reclassification of phenotypically identified staphylococcus intermedius strains. J Clin Microbiol. 2007;45:2770–8. DOIPubMedGoogle Scholar
- Clinical Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals (VET01S), 5th edition. Wayne (PA): The Institute; 2020.
- Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing (M100), 31st edition. Wayne, (PA): The Institute; 2021.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA), laboratory testing, 2019 [cited 2023 Mar 2]. https://www.cdc.gov/mrsa/lab/index.html
- Kansas Department of Health and Environment. Reportable diseases in Kansas. May 11, 2018 [cited 2023 Mar 2]. https://www.kdhe.ks.gov/DocumentCenter/View/13900/Kansas-Notifiable-Disease-List-PDF
- Missouri Department of Health and Senior Services. Diseases and conditions reportable in Missouri (19 CSR 20–20.020). May 2016 [cited 2023 Mar 2]. https://health.mo.gov/living/healthcondiseases/communicable/communicabledisease/pdf/reportablediseaselist2.pdf
- Missouri Department of Health and Senior Services. CRE reporting. December 2018 [cited 2023 Mar 2]. https://health.mo.gov/living/healthcondiseases/communicable/communicabledisease/pdf/cre-case-definitions.pdf
- Kansas Department of Agriculture. Kansas animal health reportable diseases, 2021 [cited 2023 Mar 2]. https://agriculture.ks.gov/docs/default-source/rc-ah-large-animal/reportable-disease-poster.pdf
- Missouri Department of Agriculture. Reportable communicable diseases [cited 2023 Mar 2]. https://agriculture.mo.gov/animals/health/disease/comdisease.php
- Kansas State University Veterinary Health Center. Information about antibiotic resistant bacteria in companion animals [cited 2023 Mar 2]. https://www.ksvhc.org/clients/ARB
- University of Missouri Veterinary Medical Diagnostic Laboratory. Additional information—antibiotic resistant bacteria in companion animals [cited 2023 Mar 2]. https://vmdl.missouri.edu/additional-information-antibiotic-resistant-bacteria-in-companion-animals
- Kansas Healthcare-Associated Infections & Antimicrobial Resistance Advisory Group. Resources [cited 2023 Mar 2] https://public.kfmc.org/sites/hai/SitePages/Resources.aspx
- Minnesota Board of Animal Health. Veterinary alert. Antimicrobial resistance (super bugs) in companion animals. March 28, 2022 [cited 2023 Mar 2]. https://content.govdelivery.com/accounts/MNBAH/bulletins/310d6a2
- National Center for Biotechnology Information. Pathogen detection isolate browser, Klebsiella [cited 2023 Mar 2]. https://www.ncbi.nlm.nih.gov/pathogens/isolates/#PRJNA325243%20AND%20klebsiella
- National Center for Biotechnology Information. Pathogen detection isolate browser, Escherichia coli [cited 2023, Mar 2]. https://www.ncbi.nlm.nih.gov/pathogens/isolates/#PRJNA325243%20AND%20escherichia
- Burke M, Santoro D. Prevalence of multidrug-resistant coagulase-positive staphylococci in canine and feline dermatological patients over a 10-year period: a retrospective study. Microbiology (Reading). 2023;169:
001300 . DOIPubMedGoogle Scholar - Morris DO, Loeffler A, Davis MF, Guardabassi L, Weese JS. Recommendations for approaches to meticillin-resistant staphylococcal infections of small animals: diagnosis, therapeutic considerations and preventative measures.: Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet Dermatol. 2017;28:304–e69. DOIPubMedGoogle Scholar
- Laarhoven LM, de Heus P, van Luijn J, Duim B, Wagenaar JA, van Duijkeren E. Longitudinal study on methicillin-resistant Staphylococcus pseudintermedius in households. PLoS One. 2011;6:
e27788 . DOIPubMedGoogle Scholar - Asleh M, Feinstein Y, Lazar I, Rokney A, Baum M, Sagi O, et al. Severe pneumonia caused by methicillin-resistant Staphylococcus pseudintermedius in an oncology patient: case report and literature review. Microb Drug Resist. 2022;28:222–8. DOIPubMedGoogle Scholar
- Davis MF, Misic AM, Morris DO, Moss JT, Tolomeo P, Beiting DP, et al. Genome sequencing reveals strain dynamics of methicillin-resistant Staphylococcus aureus in the same household in the context of clinical disease in a person and a dog. Vet Microbiol. 2015;180:304–7. DOIPubMedGoogle Scholar
- Waltenburg MA, Shugart A, Loy JD, Tewari D, Zhang S, Cole SD, et al.; CRO Veterinary Diagnostic Laboratory Investigation Group. A survey of current activities and technologies used to detect carbapenem resistance in bacteria isolated from companion animals at veterinary diagnostic laboratories—United States, 2020. J Clin Microbiol. 2022;60:
e0215421 . DOIPubMedGoogle Scholar - Weese JS, Blondeau JM, Boothe D, Breitschwerdt EB, Guardabassi L, Hillier A, et al. Antimicrobial use guidelines for treatment of urinary tract disease in dogs and cats: antimicrobial guidelines working group of the international society for companion animal infectious diseases. Vet Med Int. 2011;2011:
263768 . DOIPubMedGoogle Scholar - Healthcare-Associated Infections & Antimicrobial Resistance Advisory Group. [cited 2023 Mar 2]. https://public.kfmc.org/sites/hai/SitePages/Home.aspx
Figures
Cite This ArticleOriginal Publication Date: May 12, 2023
1Current affiliation: National Bio and Agro-Defense Facility, Manhattan, Kansas, USA.
2Current affiliation: Kansas Department of Agriculture, Manhattan, Kansas, USA.
3Current affiliation: Kansas Intelligence Fusion Center, Topeka, Kansas, USA.
Table of Contents – Volume 29, Number 6—June 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Kate KuKanich, Department of Clinical Sciences, College of Veterinary Medicine, Kansas State University, 1800 Denison Ave, Manhattan, KS 66506, USA
Top