Volume 29, Number 6—June 2023
Dispatch
SARS-CoV-2 Seroprevalence and Cross-Variant Antibody Neutralization in Cats, United Kingdom
Cite This Article
Citation for Media
Abstract
Anthropogenic transmission of SARS-CoV-2 to pet cats highlights the importance of monitoring felids for exposure to circulating variants. We tested cats in the United Kingdom for SARS-CoV-2 antibodies; seroprevalence peaked during September 2021–February 2022. The variant-specific response in cats trailed circulating variants in humans, indicating multiple human-to-cat transmissions over a prolonged period.
The World Organisation for Animal Health reported that 26 different animal species had been infected with SARS-CoV-2 by December 31, 2022; ≈30% (8/26) of the susceptible species are felids (1). Animal SARS-CoV-2 infections originating from anthropogenic transmission can lead to onward animal-to-animal transmission, as described previously in mink (2), hamsters (3), and white-tailed deer (4). There have also been reports of animal-to-human transmission of SARS-CoV-2 from farmed mink (2), pet hamsters (5), free-ranging white-tailed deer (6), and a pet cat (7).
It is unknown whether individual SARS-CoV-2 variants are more or less likely to be transmitted from humans to cats or whether infected cats are more or less likely to develop clinical signs. The aim of this study was to assess the seroprevalence of SARS-CoV-2 infection in cats during April 2020–February 2022 in the United Kingdom. We used a pseudotype-based neutralization assay (PVNA) to measure virus neutralizing antibody titers and a confirmatory ELISA that measured antibodies recognizing the receptor binding domain of the SARS-CoV-2 spike (S) protein. We measured neutralizing titers against a panel of viral pseudotypes based on a lentiviral (HIV) backbone and bearing the S proteins of the predominant circulating variants in the United Kingdom to investigate the specificity of the neutralizing response. The University of Glasgow Veterinary Ethics Committee granted approval for the study (EA27/20).
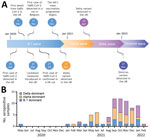
We screened residual blood samples from 2,309 cats by using PVNA at a final dilution of 1:100; the samples were submitted to the University of Glasgow Veterinary Diagnostic Services laboratory (VDS) during April 2020–February 2022 (Figure 1, panel A). The samples represented a cohort that was broadly representative of the domestic cat population in the United Kingdom, including samples from 112 of the 126 UK postcode areas (Appendix 1 Figure 1), although the samples had an uneven distribution unrelated to the local human population density. Overrepresented areas included Blackpool, Glasgow, Edinburgh, and Cambridge. The PVNA used HIV (SARS-CoV-2) pseudotypes bearing S proteins of SARS-CoV-2 ancestral D614G (B.1), Alpha (B.1.1.7), Delta (B.1.617.2) or Omicron (BA.1). Samples submitted early in the pandemic were tested against ancestral D614G (B.1) only, whereas new variants were included as they emerged (Appendix 2). We estimated neutralization titers for positive samples by performing the PVNA with serially diluted samples.
Our results showed that SARS-CoV-2 seroprevalence in UK cats increased over time (Figure 1, panel B). Overall, the seroprevalence during the study period was 3.2% (95% CI 2.56%–4.05%; 75/2,309). Seroprevalence was highest during September–November 2021 (5.3%, 95% CI 3.69%–7.23%; 35/666) and during December 2021–February 2022 (5.2%, 95% CI 3.09%–8.05%; 18/348).
When we analyzed individual samples, we observed differences in variant-specific potencies among titers against the different SARS-CoV-2 variants: 17/75 (22.7%) samples were B.1 dominant (i.e., they possessed higher titers against B.1 than against other variants); 31/75 (41.3%) were Alpha dominant, and 27/75 (36%) were Delta dominant. On average, Delta-dominant samples displayed higher neutralization titers (mean 760) against their dominant pseudotype compared with Alpha-dominant (488; p = 0.06) or B.1-dominant (329; p = 0.02) samples (Appendix 1 Figure 2). Throughout the study period (April 2020–February 2022), no Omicron-dominant seropositive samples were identified; we anticipated this finding because only a small proportion of samples were collected after the Omicron variant emerged.
We observed an association between the dominant variant in cats and the timeline of variant emergence in the human population. Detection of new dominant variants in cats trailed detection of the variant in the humans; however, we detected dominant titers against extinct variants even after human cases had declined, possibly indicating long-lasting humoral immunity (Figure 2). We observed 3 distinct patterns of neutralization. B.1-dominant samples generally had slightly lower titers against the Alpha pseudotype than against B.1. Those samples also had significantly lower titers against both the Delta (p<0.0001) and Omicron (p<0.001) pseudotypes. Alpha-dominant samples showed slightly lower B.1 titers and markedly lower Delta and Omicron titers. Delta-dominant samples showed similar titers against the B.1, Alpha, and Omicron pseudotypes, all of which were significantly lower than their Delta titers (p<0.0001) (Appendix 1 Figure 3).
The trends we observed for cats thought to have been infected with the B.1 variant are similar to the patterns of neutralization in humans reported previously (8); Wilhelm et al. showed that humans vaccinated with an ancestral strain–based vaccine develop lower neutralization titers against the Delta and Omicron variants than against B.1 or Alpha. Another study showed that cats experimentally inoculated with either the ancestral or the Delta variant became lethargic and pyrexic, whereas Omicron-inoculated cats did not develop any clinical signs and displayed lower levels of virus shedding, suggesting that the Omicron variant might be less pathogenic in cats as well as in humans (9).
Despite those distinct patterns of neutralization, the variant to which the animal was exposed can only be speculatively inferred through serologic testing in the absence of viral sequence data, even in cases in which the titer against the dominant variant is many times greater than the next highest titer. The 3 specific patterns of immunity we observed were similar to previous findings in humans (10). It is likely that both the antigenicity of the different variants’ S proteins and the viral load during the infection period influence the breadth and potency of variant-specific neutralization.
A greater proportion of purebred cats (31/720 [4.3%, 95% CI 2.94%–6.06%]) than nonpedigree cats (39/1,300 [3%, 95% CI = 2.14%–4.08%]) were seropositive; however, this finding was not significant (p = 0.1). Purebred cats are more likely to be kept indoors only and may therefore experience more close contact with their owners, meaning they are more prone to exposure to SARS-CoV-2 if their owners become infected.
Although a definitive protective threshold antibody level for SARS-CoV-2 has not yet been established, waning neutralizing antibody levels in humans after vaccination have been associated with reinfection and reduced protection against novel variants (11). Sequential samples >12 days apart were collected from 5 seropositive cats. In all 5 cases, the neutralizing titers against SARS-CoV-2 waned over time. The average percentage decrease in titer per day was highly variable across samples, although for 3 of 5 cats it was consistent across all variants (Table).
This study demonstrated increasing seroprevalence of SARS-CoV-2 antibodies in the UK domestic cat population, consistent with results reported in a survey of cats and dogs recently conducted in Canada (12) and the low seroprevalence observed during the first and second waves of the pandemic (13,14). This increase could be explained by the persistence of the humoral response over time, with a consequent accumulation in the number of seropositive results in the population. In addition, increased seroprevalence during the later months of the pandemic may mean the likelihood of human-to-cat transmission is greater for newer variants that have previously been shown to be more readily transmitted between humans (15), although this hypothesis has not been confirmed experimentally.
This study demonstrates the importance of adopting a One Health approach to monitor SARS-CoV-2 infections in pet cats that are in close contact with their SARS-CoV-2–positive owners. Changes in transmissibility of emerging variants should be monitored in cats as well as humans.
This article was preprinted at https://www.biorxiv.org/content/10.1101/2022.11.18.517046v1.
Miss Tyson is a PhD candidate at the MRC-University of Glasgow Centre for Virus Research, Glasgow, Scotland. Her primary research interests include viral immunology, humoral immunity, and viruses at the human–animal interface.
Acknowledgments
We thank Dawn Dunbar and Andrea Bowie for assisting with sample provision.
UK Research and Innovation—Biotechnology and Biological Sciences Research Council (UKRI-BBSRC) contributed to the funding of this research.
References
- World Organisation for Animal Health. COVID-19—events in animals. 2022 [cited 2022 May 1]. https://www.oie.int/en/what-we-offer/emergency-and-resilience/covid-19/#ui-id-3
- Oreshkova N, Molenaar RJ, Vreman S, Harders F, Oude Munnink BB, Hakze-van der Honing RW, et al. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Euro Surveill. 2020;25:
2001005 . DOIPubMedGoogle Scholar - Sia SF, Yan LM, Chin AWH, Fung K, Choy KT, Wong AYL, et al. Pathogenesis and transmission of SARS-CoV-2 in golden hamsters. Nature. 2020;583:834–8. DOIPubMedGoogle Scholar
- Kuchipudi SV, Surendran-Nair M, Ruden RM, Yon M, Nissly RH, Vandegrift KJ, et al. Multiple spillovers from humans and onward transmission of SARS-CoV-2 in white-tailed deer. Proc Natl Acad Sci U S A. 2022;119:
e2121644119 . DOIPubMedGoogle Scholar - Yen HL, Sit THC, Brackman CJ, Chuk SSY, Gu H, Tam KWS, et al.; HKU-SPH study team. Transmission of SARS-CoV-2 delta variant (AY.127) from pet hamsters to humans, leading to onward human-to-human transmission: a case study. Lancet. 2022;399:1070–8. DOIPubMedGoogle Scholar
- Pickering B, Lung O, Maguire F, Kruczkiewicz P, Kotwa JD, Buchanan T, et al. Divergent SARS-CoV-2 variant emerges in white-tailed deer with deer-to-human transmission. Nat Microbiol. 2022;7:2011–24. DOIPubMedGoogle Scholar
- Sila T, Sunghan J, Laochareonsuk W, Surasombatpattana S, Kongkamol C, Ingviya T, et al. Suspected cat-to-human transmission of SARS-CoV-2, Thailand, July–September 2021. Emerg Infect Dis. 2022;28:1485–8. DOIPubMedGoogle Scholar
- Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, et al. Limited neutralisation of the SARS-CoV-2 Omicron subvariants BA.1 and BA.2 by convalescent and vaccine serum and monoclonal antibodies. EBioMedicine. 2022;82:
104158 . DOIPubMedGoogle Scholar - Martins M, do Nascimento GM, Nooruzzaman M, Yuan F, Chen C, Caserta LC, et al. The Omicron variant BA.1.1 presents a lower pathogenicity than B.1 D614G and Delta variants in a feline model of SARS-CoV-2 infection. J Virol. 2022;96:
e0096122 . DOIPubMedGoogle Scholar - Manali M, Bissett LA, Amat JAR, Logan N, Scott S, Hughes EC, et al. SARS-CoV-2 evolution and patient immunological history shape the breadth and potency of antibody-mediated immunity. J Infect Dis. 2022;227:40–9. DOIPubMedGoogle Scholar
- Ahmed S, Mehta P, Paul A, Anu S, Cherian S, Shenoy V, et al. Postvaccination antibody titres predict protection against COVID-19 in patients with autoimmune diseases: survival analysis in a prospective cohort. Ann Rheum Dis. 2022;81:868–74. DOIPubMedGoogle Scholar
- Bienzle D, Rousseau J, Marom D, MacNicol J, Jacobson L, Sparling S, et al. Risk factors for SARS-CoV-2 infection and illness in cats and dogs. Emerg Infect Dis. 2022;28:1154–62. DOIPubMedGoogle Scholar
- Smith SL, Anderson ER, Cansado-Utrilla C, Prince T, Farrell S, Brant B, et al. SARS-CoV-2 neutralising antibodies in dogs and cats in the United Kingdom. Curr Res Virol Sci. 2021;2:
100011 . DOIPubMedGoogle Scholar - Mahase E. Delta variant: what is happening with transmission, hospital admissions, and restrictions? BMI. 2021;373:n1513.
- Torjesen I. Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. 2021;375:n2943. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: April 26, 2023
Table of Contents – Volume 29, Number 6—June 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Grace Tyson, Henry Wellcome Building, Rm 434, Garscube, Glasgow, G61 1QH Scotland, UK
Top