Volume 30, Number 11—November 2024
Research
Quantitative SARS-CoV-2 Spike Receptor-Binding Domain and Neutralizing Antibody Titers in Previously Infected Persons, United States, January 2021–February 2022
Figure 1
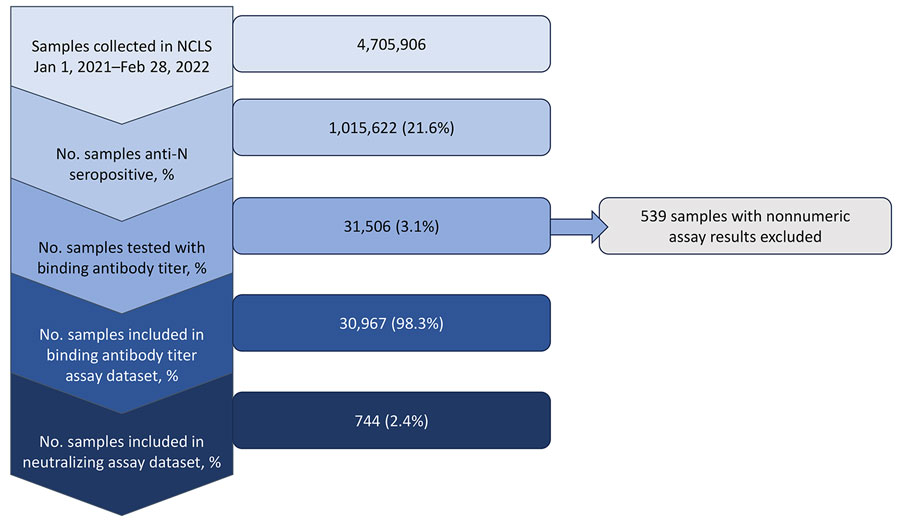
Figure 1. Flowchart of sample selection in study of quantitative SARS-CoV-2 spike receptor-binding domain and neutralizing antibody titers in previously infected persons, United States, January 2021–February 2022. Nonnumeric results are quantitative receptor-binding domain assays with invalid results due to insufficient serum volume, lost sample, or poor reproducibility. NCLS, Nationwide Commercial Laboratory Seroprevalence Survey.
1These co-senior authors contributed equally to this article.
Page created: September 30, 2024
Page updated: October 22, 2024
Page reviewed: October 22, 2024
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.