Volume 30, Number 11—November 2024
Research
Quantitative SARS-CoV-2 Spike Receptor-Binding Domain and Neutralizing Antibody Titers in Previously Infected Persons, United States, January 2021–February 2022
Figure 5
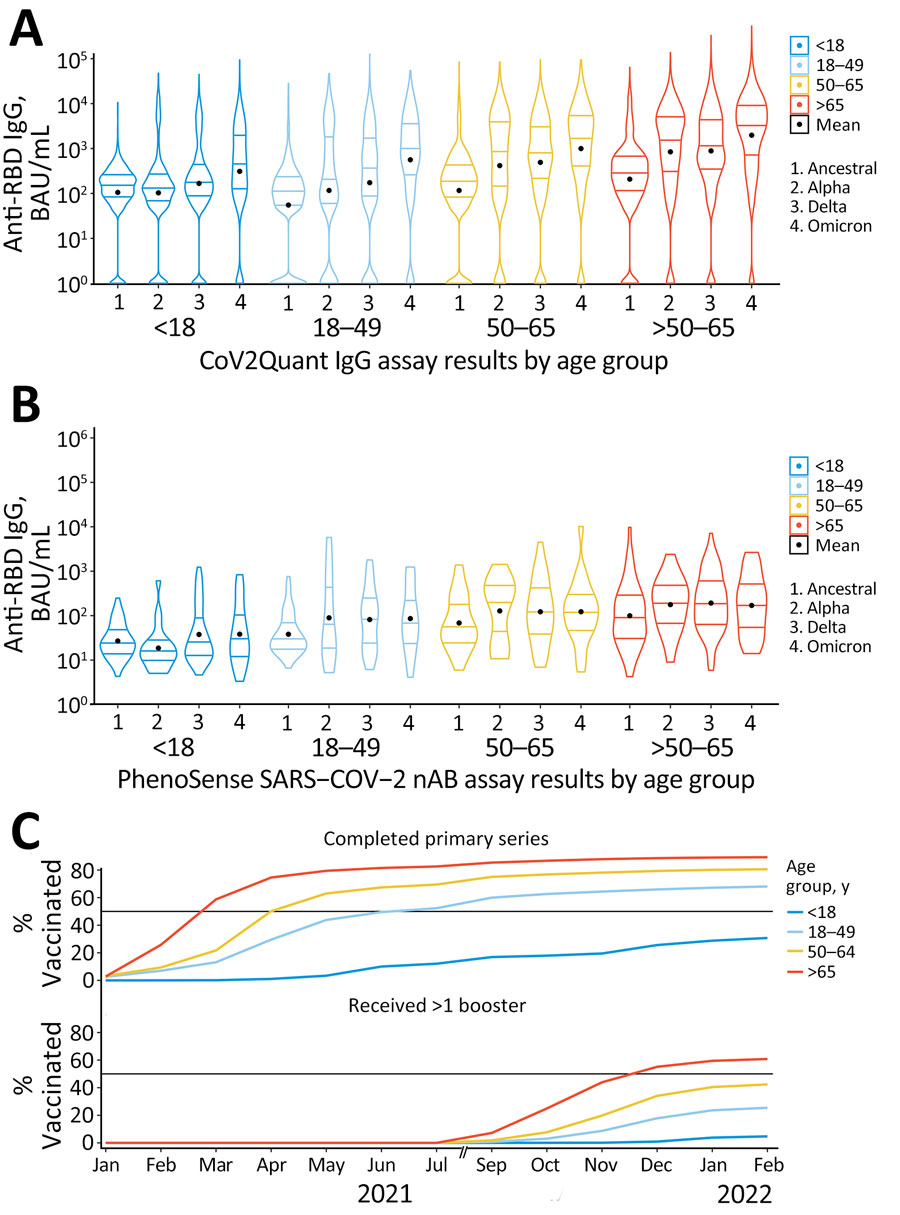
Figure 5. Violin plots of anti-RBD IgG and NT50 titer results by age group in study of quantitative SARS-CoV-2 spike RBD and neutralizing antibody titers in previously infected persons, United States, January 2021–February 2022. A) Anti-RBD, n = 30,967; B) NT50, n = 744; C) vaccination coverage per SARS-CoV-2 epoch. Anti-RBD measured by Cov2Quant IgG (LabCorp, https://www.labcorp.com) and NT50 measured by PhenoSense SARS-CoV-2 Neutralizing Antibody Assay (Monogram Biosciences, https://monogrambio.labcorp.com). Horizontal lines in plots indicate first quantile, median, and third quantile; black dots indicates means. Persons >16 years of age were eligible for vaccination starting in December 2020. In May 2021, vaccination was approved for persons 12–15 years of age. In November 2021, vaccination was approved for persons 5–11 years of age. Note: August 2021 is omitted due to a gap in data collection. BAU, binding antibody units; NT50, 50% neutralization titer; RBD, receptor-binding domain.
1These co-senior authors contributed equally to this article.