Volume 30, Number 12—December 2024
Dispatch
Transboundary Movement of Yezo Virus via Ticks on Migratory Birds, Japan, 2020–2021
Cite This Article
Citation for Media
Abstract
Migratory birds carry ticks harboring various pathogens, including the zoonotic Yezo virus. In Hokkaido, Japan, we collected ticks from migratory birds during 2020–2021. Eight of 385 pools, comprising 2,534 ticks, tested positive for Yezo virus RNA, suggesting Yezo virus might be spread through the flyways of migratory birds.
Yezo virus (YEZV), in the order Bunyavirales, family Nairoviridae, genus Orthonairovirus, possesses a negative-sense single-stranded RNA genome comprising 3 segments: large (L), medium (M), and small (S) (1). Each segment contains a single open reading frame encoding the RNA-dependent RNA polymerase (L segment), glycoprotein precursor (M segment), and nucleoprotein (S segment) (1). YEZV infection is an emerging infectious disease, detected in Hokkaido, Japan, in 2019 among patients who had febrile illness after a tick bite (1); >9 patients infected with YEZV have been reported in Hokkaido (1–3). Among those patients, fever, myalgia, thrombocytopenia, leukopenia, and increasing liver enzymes were commonly reported after a tick bite (1–3). One patient who had a YEZV infection after a tick bite has also reported in Inner Mongolia in northeastern China (4).
Migratory birds carry ticks harboring various pathogens, such as Crimean-Congo hemorrhagic fever virus, belonging to the family Nairoviridae (5–7). Phylogenetic analysis of tickborne severe fever with thrombocytopenia syndrome virus indicated that this virus might be carried by ticks found on migratory birds that fly between China, South Korea, and Japan (8,9). Increasing evidence suggests that migratory birds play a critical role in spreading tickborne pathogens. In archipelagos, such as Japan, understanding transmission of pathogens by migratory birds is critical; however, information on tickborne pathogens carried by migratory birds is lacking. We investigated the prevalence of YEZV in ticks found on migratory birds in Japan to determine virus transmission pathways.
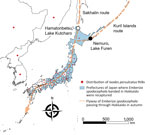
We conducted this research under approval by the animal research review board of Yamashina Institute for Ornithology, Chiba, Japan (approval nos. 2020-004 and 2021-002). We collected ticks infesting birds that mostly fly from Sakhalin and the Kuril Islands to Hokkaido, Japan. We collected the ticks during October 2–12, 2020, and October 2–13, 2021, in Lake Kutcharo, Hamatonbetsu Town, and Lake Furen, Nemuro City, Japan (Figure 1). We analyzed YEZV genes in the ticks to clarify virus spread. Because migratory birds at these locations in autumn are considered to have just arrived from Sakhalin (Sakhalin route) and the Kuril Islands (Kuril Islands route) (Figure 1), it is likely that the ticks crossed the sea attached to the birds.
We collected 2,534 ticks from 15 species of birds in October 2020 and 2021 (Appendix Tables 1, 2). All ticks were morphologically identified and pooled according to their species, life stage, and host species. Each pool consisted of <10 larval or 5 nymphal ticks (Appendix).
We examined 2,323 Ixodes persulcatus ticks (1,727 larvae and 596 nymphs), 203 I. pavlovskyi ticks (169 larvae and 34 nymphs), 1 I. turdus tick (nymph), 3 Haemaphysalis megaspinosa ticks (larvae), and 4 H. concinna ticks (3 larvae and 1 nymph) by using quantitative reverse transcription PCR (Appendix). Of those, 8 pools of I. persulcatus ticks were positive for YEZV RNA; no pools for the other tick species were YEZV positive (overall minimum infection rate 0.3%) (Table 1). Seven of 8 positive pools showed low cycle threshold (Ct) values of 22.08–30.08; 1 pool had a high Ct value of 39.19. Seven pools with low Ct values were used for further analysis. Ticks in all positive pools were I. persulcatus collected from black-faced buntings (Emberiza spodocephala) (Table 2). Five pools were collected in Lake Furen, Nemuro City, Hokkaido, and the other pools were collected in Lake Kutcharo, Hamatonbetsu Town, Hokkaido. Results indicated that ticks with YEZV were transferred by migratory birds along both the Sakhalin and Kuril Islands routes.
In I. persulcatus ticks, the minimum infection rate of YEZV was 0.2% in larvae and 0.8% in nymphs (Table 1). YEZV has been reported in 3 adult tick species, H. megaspinosa, I. ovatus, and I. persulcatus, found on vegetation in Hokkaido (1). In addition, 4 YEZV–positive pools consisted of only unfed ticks (Appendix Table 3), indicating that I. persulcatus might transmit Yezo virus.
We sequenced 7 complete genomes of YEZV: YEZV/tick/BT-1821/Japan/2020, YEZV/tick/BT-1826/Japan/2020, YEZV/tick/BT-1844/Japan/2020, YEZV/tick/BT-1864/Japan/2020, YEZV/tick/BT-1968/Japan/2020, YEZV/tick/BT-2135/Japan/2021, and YEZV/tick/BT-2155/Japan/2021 (Appendix) and deposited those sequences into the DNA Data Bank of Japan (https://www.ddbj.nig.ac.jp) (Table 2). For 1 pool that had a high Ct value, YEZV/tick/BT-1822/Japan/2020, we could not determine the complete genome sequence; however, we amplified a short fragment of the S segment by nested reverse transcription PCR and deposited that sequence in the DNA Data Bank of Japan (Table 2).
We analyzed the nucleotide and amino acid sequences of the RNA-dependent RNA polymerase, glycoprotein precursor, and nucleoprotein among YEZV strains and compared those sequences to Sulina virus IxriSL16–01 sequences (10) (Appendix Tables 4–6). Sequence identity between YEZV strains was 93.0%–100.0% at the nucleotide level and 99.2%–100.0% at the amino acid level.
We also performed phylogenetic analysis of YEZV strains by using the nucleotide sequences of the open reading frames of the L, M, and S segments (Figure 2). All tick-derived strains detected in this study belonged to the same cluster as the strains obtained from patients in Hokkaido, YEZV/human/HH003-2020/Japan/2020 and YEZV/human/HH008-2017/Japan/2017 (1). In addition, our tick-derived strain, YEZV/tick/BT-2135/Japan/2021, and the tick-derived strain from Heilongjiang, China, YEZV/tick/T-HLJ02/People’s Republic of China/2021, were reassortment strains; both were generated through reassortment with the strains found in patients in Hokkaido, Japan. We also confirmed the events of genetic reassortment by using a recombination detection program (A. Nishino and K. Maeda, unpub. data), which suggested that YEZV might be transferred between China and Hokkaido, Japan.
We successfully detected YEZV in I. persulcatus ticks from migratory birds (E. spodocephala buntings) flying to Hokkaido, Japan, from Sakhalin and the Kuril Islands (5,11). The distribution of I. persulcatus ticks partially overlapped with the flyway of E. spodocephala buntings passing through Hokkaido in autumn (12-15). Together with the possible genetic reassortment event between YEZV strains from Japan and China, this finding indicated that YEZV might be carried by migratory birds from other countries to Japan and from Hokkaido to other prefectures in Japan. To elucidate the distribution area and transmission routes of YEZV, further surveillance of YEZV infection should be conducted in ticks, birds, and wild and domestic animals.
Ms. Nishino is a PhD candidate at the Joint Graduate School of Veterinary Medicine, Yamaguchi University, and a researcher at the Department of Veterinary Science, National Institute of Infectious Diseases. Her primary research interest focuses on transboundary tickborne diseases carried by migratory birds.
Acknowledgments
We thank Asako Shigeno for analyzing nucleotide sequences and Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant no. 20H00652), the Japan Agency for Medical Research and Development (AMED) (grant nos. JP20wm0225009, JP23wm0225935, JP21fk0108613, JP22fk0108625, JP22fk0108634, JP23fk0108644, and JP23fk0108625), and the Environment Research and Technology Development Fund (grant no. 4-2005).
References
- Kodama F, Yamaguchi H, Park E, Tatemoto K, Sashika M, Nakao R, et al. A novel nairovirus associated with acute febrile illness in Hokkaido, Japan. Nat Commun. 2021;12:5539. DOIPubMedGoogle Scholar
- Suzuki K, Suzuki S, Yamaguchi H, Kakinoki Y. Tick-borne disease with Yezo virus and Borrelia miyamotoi coinfection. Intern Med. 2024;63:2861–4. DOIPubMedGoogle Scholar
- Ogata Y, Sato T, Kato K, Kikuchi K, Mitsuhashi K, Watari K, et al. A case of tick-borne Yezo virus infection: Concurrent detection in the patient and tick. Int J Infect Dis. 2024;143:
107038 . DOIPubMedGoogle Scholar - Lv X, Liu Z, Li L, Xu W, Yuan Y, Liang X, et al. Yezo virus infection in tick-bitten patient and ticks, northeastern China. Emerg Infect Dis. 2023;29:797–800. DOIPubMedGoogle Scholar
- Ishiguro F, Takada N, Masuzawa T, Fukui T. Prevalence of Lyme disease Borrelia spp. in ticks from migratory birds on the Japanese mainland. Appl Environ Microbiol. 2000;66:982–6. DOIPubMedGoogle Scholar
- Waldenström J, Lundkvist A, Falk KI, Garpmo U, Bergström S, Lindegren G, et al. Migrating birds and tickborne encephalitis virus. Emerg Infect Dis. 2007;13:1215–8. DOIPubMedGoogle Scholar
- Lindeborg M, Barboutis C, Ehrenborg C, Fransson T, Jaenson TGT, Lindgren PE, et al. Migratory birds, ticks, and crimean-congo hemorrhagic fever virus. Emerg Infect Dis. 2012;18:2095–7. DOIPubMedGoogle Scholar
- Yoshikawa T, Shimojima M, Fukushi S, Tani H, Fukuma A, Taniguchi S, et al. Phylogenetic and geographic relationships of severe fever with thrombocytopenia syndrome virus in China, South Korea, and Japan. J Infect Dis. 2015;212:889–98. DOIPubMedGoogle Scholar
- Yun Y, Heo ST, Kim G, Hewson R, Kim H, Park D, et al. Phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in South Korea and migratory bird routes between China, South Korea, and Japan. Am J Trop Med Hyg. 2015;93:468–74. DOIPubMedGoogle Scholar
- Tomazatos A, von Possel R, Pekarek N, Holm T, Rieger T, Baum H, et al. Discovery and genetic characterization of a novel orthonairovirus in Ixodes ricinus ticks from Danube Delta. Infect Genet Evol. 2021;88:
104704 . DOIPubMedGoogle Scholar - BirdLife International. East Asia/Australasia Flyway [cited 2023 Feb 26]. https://datazone.birdlife.org/userfiles/file/sowb/flyways/8_East_Asia_Australasia_Factsheet.pdf
- St John HK, Masuoka P, Jiang J, Takhampunya R, Klein TA, Kim HC, et al. Geographic distribution and modeling of ticks in the Republic of Korea and the application of tick models towards understanding the distribution of associated pathogenic agents. Ticks Tick Borne Dis. 2021;12:
101686 . DOIPubMedGoogle Scholar - Yamashina Institute for Ornithology. Atlas of Japanese migratory birds from 1961 to 1995 [in Japanese] [cited 2024 Nov 18]. https://www.biodic.go.jp/banding_en/banding/atlas.html
- Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from ixodes ticks. Clin Microbiol Rev. 2006;19:708–27. DOIPubMedGoogle Scholar
- Takada N, Takahashi M, Fujita H, Natsuaki M. Tick & tick-borne infectious diseases. In: Takada N, editor. Medical acarology in Japan [in Japanese]. Tokyo: Hokuryukan; 2019. p. 114–249.
Figures
Tables
Cite This ArticleTable of Contents – Volume 30, Number 12—December 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Ken Maeda, Department of Veterinary Science, National Institute of Infectious Diseases, 1-23-1 Toyama, Shinjuku-ku, Tokyo 162-8640, Japan
Top