Volume 30, Number 2—February 2024
Dispatch
Identification of Large Adenovirus Infection Outbreak at University by Multipathogen Testing, South Carolina, USA, 2022
Cite This Article
Citation for Media
Abstract
Using multipathogen PCR testing, we identified 195 students with adenovirus type 4 infections on a university campus in South Carolina, USA, during January–May 2022. We co-detected other respiratory viruses in 43 (22%) students. Continued surveillance of circulating viruses is needed to prevent virus infection outbreaks in congregate communities.
Human adenovirus (HAdV) infections can cause a range of symptoms but most commonly result in respiratory illnesses (1). Most HAdV infections are not clinically severe; however, more serious illness can occur (2,3). A total of 51 recognized HAdV serotypes and >100 genotypes (classified into 7 species, HAdV-A–G) have been characterized globally (4). Because testing does not change clinical management, persons with HAdV infections often do not receive a virus infection diagnosis. If adenovirus testing is available, it is usually performed as part of a multipathogen PCR panel. Adenovirus infection outbreaks caused by transmission through respiratory droplets and fomites have been reported in various congregate settings, including nursing homes (5), military recruit barracks (6–8), and college campuses (9–11). The incubation period varies from 2–14 days.
In early February 2022, a university campus in South Carolina, USA, notified its regional health department of 4 students with HAdV infections who had sought care for respiratory symptoms the previous week at student health services (SHS). Nasopharyngeal swab specimens were collected and tested by using a multipathogen PCR panel for respiratory pathogens; HAdV was detected in all 4 patient samples. SHS contacted the South Carolina Department of Health and Environmental Control and the Centers for Disease Control and Prevention (CDC) to request typing of the HadV specimens to determine if >1 HadV type was circulating. Partial genomic sequencing showed that HadV was the same type in all 4 specimens. Cases of HadV infections continued to increase on campus; therefore, the university, state and local health departments, and CDC investigated the scope of the outbreak. The timing of this outbreak during the COVID-19 pandemic enabled unique observations and responses. We describe the outbreak, the university’s response intended to prevent further transmission, and implications of HadV infection outbreaks in congregate settings, such as universities. The South Carolina Department of Health and Environmental Control Institutional Review Board deemed this work was non–human subjects research. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.
We analyzed laboratory and exposure data from symptomatic students who sought care at the university’s health clinic during January 1–May 31, 2022. SHS staff collected nasal or nasopharyngeal swab samples from students and tested those specimens by using BioFire RSP 2.1 multiplex PCR (bioMérieux, https://www.biomerieux.com). We defined positive cases as students who manifested respiratory or constitutional symptoms and were HadV-positive in the multipathogen PCR panel.
SHS routinely collected demographic information, symptoms, and medical history from students seeking care at the university clinic. Those data were supplemented from March 22–May 10, 2022, by using focused call-back interviews of students who had confirmed HadV infections to identify potential transmission events and locations. We used the aggregate interview data to examine student behaviors and activities that might have been associated with transmission events.
We considered students who tested positive for HadV plus another respiratory pathogen on the multipathogen panel to have pathogen co-detections. We compared demographic characteristics, symptoms, and illness severity between students who had co-detections and those who were only infected with HadV by using t-tests.
During January 1–May 31, 2022, a total of 687 students were tested by using the respiratory multipathogen PCR panel after seeking care at SHS for acute respiratory or systemic symptoms. Of those 687 students, 195 (28.4%) tested positive for HadV; HadV infections were distributed evenly between men and women. The median age of infected students was 19 years (range 18–24 years) (Table 1). The most common symptoms reported by students were sore throat (85%), cough (76%), fever (75%), and headache (62%). Nausea and vomiting were reported by 27% of students; conjunctivitis was reported by 10% of students. No known emergency department visits, hospitalizations, or deaths were reported among any HadV-infected students. HadV-4 was identified in 30 swab specimens by partial genomic sequencing of the hexon gene (Appendix). We randomly selected 8 of those 30 sequences for whole-genome sequencing and performed phylogenetic analyses (Appendix Figure).
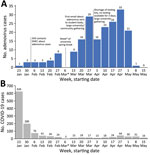
Weekly case counts increased slowly during January–February and more rapidly after the week of March 6 (university spring break) (Figure). Rapid and detailed attention to surface decontamination in academic and residential buildings on campus was reported in February and early March. Mandatory masking recommendations as part of COVID-19 mitigation efforts were lifted in March. Cases fell precipitously after students left campus for summer break (week of May 1); no cases were reported after May 10, 2022. The outbreak investigation was closed on June 7 after 2 full incubation periods (total of 28 days).
Of all HadV-infected students, infections occurred most in first-year (39%) and second-year (30%) students. Most (115 [59%]) students lived in on-campus dormitories. Three of 22 affected dormitories had >10 students with confirmed HadV infections during the outbreak. We did not observe a preponderance of one academic area of study that might suggest clustering according to academic departments.
Among the 195 students who tested positive for HadV, >1 other respiratory pathogen was also detected in 43 (22%) students (Table 2). The most common co-detected viruses were human rhinovirus/enterovirus (28 [65%]), seasonal coronaviruses (9 [21%]), and SARS-CoV-2 (8 [19%]). Most (42 [98%]) HadV-infected students who had co-detected viruses reported a sore throat, compared with 124 (82%) students infected with only HadV (p = 0.001). More HadV-only–infected students (13%) reported conjunctivitis than students who had co-detected viruses (2%; p = 0.001). We did not observe a substantial difference in disease severity (i.e., need for intravenous fluids) among HadV-only–infected students compared with those who had virus co-detections (4% vs. 2%; p = 0.07).
The university clinic staff began interviewing students with confirmed HadV infection on March 21. Interviews were completed for 96 of 121 students with HadV (79% response rate) before the end of the outbreak. Most (62 [65%]) students did not know where they had acquired infection; 9 (9%) students had a known exposure to someone with confirmed HadV infection, and 18 (19%) students had exposure to someone with similar symptoms before illness onset. Seventy-five (78%) students had not traveled away from campus before their symptoms began. We were unable to link infections to campus or off-campus locations because of limited sample size.
This outbreak of respiratory illness attributed to HadV-4 was among the largest described HadV outbreaks on a university campus (9,12); symptoms and transmission were similar to other large HadV outbreaks in congregate settings (13). Infected students were mostly freshmen and sophomores living in dormitories, highlighting increased transmission in close university settings. The outbreak occurred during the COVID-19 pandemic, and detection of HadV might have been delayed without availability of multipathogen testing. The outbreak on campus appeared to end when student density decreased during summer break, and further transmission was not observed among the limited number of students remaining on campus.
SHS was able to detect and respond quickly to a potentially serious virus infection outbreak by using multipathogen testing. Furthermore, this testing enabled identification of students who were infected with multiple pathogens. In addition to SARS-CoV-2, many other respiratory viruses can be detected in university students, including those that cause illness and outbreaks. As universities move beyond COVID-19 as the main public health priority affecting students and campuses, renewed attention to other pathogens is needed (14). Continued surveillance of circulating viruses in congregate communities remains critical for ongoing risk communication and prevention efforts.
Dr. Tori is a career epidemiology field officer in the Division of State and Local Readiness, Office of Readiness and Response, Centers for Disease Control and Prevention, Atlanta, Georgia, USA, stationed in South Carolina. This work was performed while he was the CDC’s Epidemic Intelligence Service officer in South Carolina. His research focuses on communicable disease prevention.
Acknowledgments
We thank Ana Endsley, William Harley, Adriana Kajon, Marie Killerby, Rachel Radcliffe, Warren Scott, and Rebecca Walker for their work in responding to the outbreak at the university.
Specimen testing at the New York State Public Health Laboratory was partially supported by the CDC’s Vaccine Preventable Disease Reference Center contract (cooperative agreement no. 5NU600E000104).
This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy (e.g., 45 Code of Federal Regulations part 46, 21 Code of Federal Regulations part 56; 42 United States Code [U.S.C.] §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq).
References
- Khanal S, Ghimire P, Dhamoon AS. The repertoire of adenovirus in human disease: the innocuous to the deadly. Biomedicines. 2018;6:30. DOIPubMedGoogle Scholar
- Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014;27:441–62. DOIPubMedGoogle Scholar
- Gutierrez Sanchez LH, Shiau H, Baker JM, Saaybi S, Buchfellner M, Britt W, et al. A case series of children with acute hepatitis and human adenovirus infection. N Engl J Med. 2022;387:620–30. DOIPubMedGoogle Scholar
- Committee on Infectious Diseases. Red book: 2021–2024 report of the Committee on Infectious Diseases, 32nd ed. Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH, editors. Itasca (IL): American Academy of Pediatrics; 2021.
- Kajon AE, Lamson DM, Bair CR, Lu X, Landry ML, Menegus M, et al. Adenovirus type 4 respiratory infections among civilian adults, northeastern United States, 2011–2015. Emerg Infect Dis. 2018;24:201–9. DOIPubMedGoogle Scholar
- Rogers AE, Lu X, Killerby M, Campbell E, Gallus L, Kamau E, et al. Outbreak of acute respiratory illness associated with adenovirus type 4 at the U.S. Naval Academy, 2016. MSMR. 2019;26:21–7.PubMedGoogle Scholar
- Ko JH, Woo HT, Oh HS, Moon SM, Choi JY, Lim JU, et al. Ongoing outbreak of human adenovirus-associated acute respiratory illness in the Republic of Korea military, 2013 to 2018. Korean J Intern Med (Korean Assoc Intern Med). 2021;36:205–13. DOIPubMedGoogle Scholar
- Chu VT, Simon E, Lu X, Rockwell P, Abedi GR, Gardner C, et al. Outbreak of acute respiratory illness associated with human adenovirus type 4 at the United States Coast Guard Academy, 2019. J Infect Dis. 2022;225:55–64. DOIPubMedGoogle Scholar
- Kujawski SA, Lu X, Schneider E, Blythe D, Boktor S, Farrehi J, et al. Outbreaks of adenovirus-associated respiratory illness on 5 college campuses in the United States, 2018–2019. Clin Infect Dis. 2021;72:1992–9. DOIPubMedGoogle Scholar
- Biggs HM, Lu X, Dettinger L, Sakthivel S, Watson JT, Boktor SW. Adenovirus-associated influenza-like illness among college students, Pennsylvania, USA. Emerg Infect Dis. 2018;24:2117–9. DOIPubMedGoogle Scholar
- Lamson DM, Kajon A, Popowich M, Fuschino M, St George K. Human adenovirus 7d strains associated with influenza-like illness, New York, USA, 2017–2019. Emerg Infect Dis. 2020;26:1047–9. DOIPubMedGoogle Scholar
- Sivan AV, Lee T, Binn LN, Gaydos JC. Adenovirus-associated acute respiratory disease in healthy adolescents and adults: a literature review. Mil Med. 2007;172:1198–203. DOIPubMedGoogle Scholar
- Tsou TP, Tan BF, Chang HY, Chen WC, Huang YP, Lai CY, et al.; Unknown Pathogen Discovery/Investigation Group. Community outbreak of adenovirus, Taiwan, 2011. Emerg Infect Dis. 2012;18:1825–32. DOIPubMedGoogle Scholar
- Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic-United States, 2020-2021. Am J Transplant. 2021;21:3481–6. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 30, Number 2—February 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Marco E. Tori, South Carolina Department of Health and Environmental Control, 2100 Bull St, Columbia, SC 29201, USA
Top