Volume 30, Number 2—February 2024
Synopsis
Parechovirus A Circulation and Testing Capacities in Europe, 2015–2021
Abstract
Parechovirus infections usually affect neonates and young children; manifestations vary from asymptomatic to life-threatening. We describe laboratory capacity in Europe for assessing parechovirus circulation, seasonality, and epidemiology. We used retrospective anonymized data collected from parechovirus infection case-patients identified in Europe during January 2015–December 2021. Of 21 laboratories from 18 countries that participated in the study, 16 (76%) laboratories with parechovirus detection capacity reported 1,845 positive samples; 12/16 (75%) with typing capability successfully identified 517 samples. Parechovirus A3 was the most common type (n = 278), followed by A1 (153), A6 (50), A4 (13), A5 (22), and A14 (1). Clinical data from 1,269 participants highlighted correlation of types A3, A4, and A5 with severe disease in neonates. We observed a wide capacity in Europe to detect, type, and analyze parechovirus data. To enhance surveillance and response for PeV outbreaks, sharing typing protocols and data on parechovirus-positive cases should be encouraged.
Parechoviruses are small, nonenveloped, single-stranded RNA viruses belonging to the large Picornaviridae family that circulate worldwide; primary infections occur mainly in children <2 years of age (1,2). Parechoviruses are transmitted by fecal-oral and respiratory routes (2,3). Most infections are asymptomatic or have mild general gastrointestinal or respiratory symptoms, but they can occasionally lead to sepsis, meningitis or other neurologic manifestations, or even death (2–6).
Nineteen human parechovirus types have been classified as species types PeV-A1–A19 (7); the most commonly reported are A1, A3, and A6 (2,3). PeV-A1 and A6 infections are generally associated with mild outcomes, but PeV-A3 can cause severe neurologic disease in infants <3 months of age (2,4–6,8,9). More recently, PeV-A4 and A5 also have been associated with severe clinical manifestations in children (10,11). Recorded genotype distribution might vary on the basis of study design, including testing strategy, geographic location, and timing of sampling, because epidemiology can differ by virus type (3). Data collected from nonpolio enterovirus (NPEV) surveillance and childhood prevalence studies showed worldwide parechovirus distribution differs by genotype; PeV-A1 is the most prevalent type in the United States, Asia, and Europe, followed by A3 and A4 (12). PeV-A6 is reported as second most common in Australia and in some studies in Europe (2,12). Additional genotypes, including A2 and A7–A19, that are rare in Europe and the United States have been reported in India, Pakistan, and Africa (12).
Parechovirus studies in Europe have mostly focused on children or specific symptoms, with no data from dedicated surveillance and limited data from the NPEV surveillance system. The lack of systematically collected data limits full understanding of the impact and circulation of parechovirus infections. Clarifying the epidemiology, clinical implications, and phylogeny of parechovirus would help laboratories and national health authorities make decisions about the clinical relevance of infections. We therefore conducted a retrospective study to assess the presence of surveillance and laboratory capacity for parechovirus detection and typing in Europe during 2015–2021. We also described the seasonality, clinical manifestations, and molecular epidemiology of parechovirus infections during the 7-year study period (2015–2021).
Data Collection
In March 2022, the European Non-polio Enterovirus Network (ENPEN) invited the national focal point agencies that constitute the European Centre for Disease Prevention and Control (ECDC) public health network, regional reference laboratories from all 30 member states within the European Union (EU), European Economic Area, the United Kingdom, and local laboratories affiliated with ENPEN to join the study. We sent a reminder letter about participation 15 days before the deadline.
We used data collected during January 1, 2015–December 31, 2021 as part of an EU survey (13). The survey included questions for each participating laboratory on their extent of and approach to parechovirus detection and surveillance and their screening policies and capacity for detection and typing. We also requested information on methods used in each laboratory (Appendix Table 1). When available, we collected anonymized aggregated data on monthly and yearly parechovirus detection, associated clinical symptoms, age group, sample type, sex, and total number of samples tested for each study year by parechovirus type (Appendix Figure 1). Each laboratory collected data from various sources, such as NPEV, acute flaccid paralysis, and influenza-like illness (ILI) surveillance; screening of hospital admissions records; and cerebrospinal fluid (CSF) samples.
For each laboratory we summarized the capacity for parechovirus detection and what triggers they used to initiate testing (Table). We included laboratories reporting the absence of parechovirus testing, to better understand the extent of testing capacity in Europe. We asked participating laboratories to share nucleotide sequences of PeV-A3 strains that had been typed; in cases of outbreaks or clusters, we requested only nonidentical (i.e., differing by ≥2%) sequences.
Data Analysis
We reported the number of parechovirus infections by month/year and country of study, and analyzed data by clinical symptoms, age group, sample type, and parechovirus type when information was available. We calculated overall parechovirus detection rate when total number of samples tested was reported. Because some laboratories did not implement parechovirus detection testing until after the study had begun, we reported proportions of positive samples for the entire 2015–2021 study period and for the specific timeframes 2015–2017 and 2018–2021. We calculated parechovirus type distribution by year, clinical symptoms, age group, sample type, and month, and calculated the proportion of detections and types of samples. We performed χ2 testing using Vassar stat (14) to compare proportions; p<0.05 indicates statistical significance.
For PeV-A3 analysis, we summarized 106 sequences with >80% completeness in viral protein (VP) 3/VP1 junction region positions 2182–2437 (as numbered in the echovirus 22 prototype sequence L02971) (Appendix Table 2). We aligned sequences using MUSCLE 3 (15) and compared them with 630 publicly available PeV-A3 nucleotide sequences from this region retrieved from GenBank database in December 2022 using sequence editor version 1.4 (16). In addition, participating laboratories provided 30 sequences from a second region in VP1 (positions 2336–3038; Appendix Table 2), which we compared with 856 available GenBank sequences. We performed neighbor-joining phylogenetic analysis (Jukes-Cantor model) and calculated maximum likelihood using the optimal substitution model, Tamura-Nei with γ correction, using MEGA package version 7 (17). When sampling dates were available, we inspected phylogenetic trees for country-specific clustering and temporal trends.
In total, 21 laboratories from 18 EU and European Economic Area member states participated in the study; 16/21 participating laboratories performed parechovirus testing (76%). Of those not testing, 1 laboratory each in the Slovak Republic and Bulgaria planned to introduce parechovirus in routine diagnostics, but the remaining 3 laboratories, in the Czech Republic, Estonia, and Hungary, had no plans to implement nationwide parechovirus testing (Table). Of the 16 laboratories performing testing, 11 (69%) provided data for 2015–2021; 2, in Norway and the United Kingdom (Scotland), provided data only for 2015–2017, and 3, in Luxemburg, Poland, and Slovenia, reported data for 2017–2021 after commencing testing.
Twelve (75%) of 16 laboratories initially performing testing reported capacity to type parechovirus-positive samples and provided type information for this study (Table). Of those, 5 performed sequencing routinely and 7 sequenced viruses only from selected clinically detected cases. Most (11/12) laboratories analyzed sequences in the VP3/VP1 junction region positions 2182–2437, but 1 laboratory, in the Netherlands, performed sequencing from the start of VP1 (positions 2336–3038). To perform the analysis of this region, we alternatively used data from Denmark, Poland, and the United Kingdom (England) because they provided data from a longer portion of the parechovirus genome that included VP1 (Appendix Table 2).
Parechovirus Detection
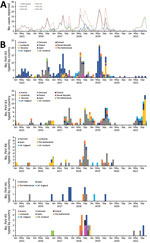
Sixteen laboratories from 13 countries reported 1,845 parechovirus-positive samples. Finland, the Netherlands, Spain, and England added parechovirus data based on voluntarily reporting positive cases to the national laboratory, to existing enterovirus surveillance (Table). Those 4 countries reported the most (65%, n = 1,200) parechovirus-positive samples. One laboratory each in Slovenia and in the Lombardy region of Italy (Italy/Lombardy) that introduced parechovirus screening into ILI surveillance provided ≈130 parechovirus-positive respiratory samples. The same laboratory in Italy/Lombardy detected parechovirus-positive samples from cases identified through the Acute Flaccid Paralysis Surveillance network, which routinely screens for polioviruses. Remaining cases were identified after clinician requests for testing not based on existing NPEV, ILI, or other surveillance systems (Table). Ireland reported the highest number of parechovirus-positive samples (26%, n = 488), followed by Denmark (17%, n = 322) and England (14%, n = 264) (Figure 1). Unfortunately, those countries provided no denominator information, so we could not calculate positivity rates.
Parechovirus testing capacity, measured by samples tested in 9 laboratories (3 in Italy and 1 each in Austria, Finland, Luxemburg, Poland, Slovenia, and the Netherlands), increased from 8,665 during 2015–2017 to 14,263 during 2018–2021; those laboratories reported 309 positive samples, 100 in 2015–2017 and 209 in 2018–2021. Although parechovirus-positive samples increased over that time, parechovirus detections per number of screened samples remained unchanged: 100/8,665 (1.3%) during 2015–2017 and 209/14,263 (1.5%) during 2018–2021. Detection rate for the entire 2015–2021 study period was 1.4% (309/22,928).
Seasonality
All participating laboratories reported month and year of collection of parechovirus-positive samples (Table; Figure 1). Infections were reported every year; 2016 accounting for 24% and 2018 for 25% of detections. Most cases were detected during June–November each year.
Distribution of Parechovirus Types
Twelve laboratories, 10 of which supplied data for the whole study period (Table), reported 517 (45%) of the 1,139 successfully sequenced parechovirus-positive samples, corresponding to 28% (517 /1,845) of all positive samples reported in this study. Among 6 parechovirus types detected, PeV-A3 (54%, n = 278) was the most frequently reported, followed by A1 (30%, n = 153), A6 (10%, n = 50), A5 (4%, n = 22), A4 (2%, n = 13), and A14 (0.2%, n = 1) (Figure 2). Positive PeV-A1 and A3 samples were reported each year during 2015–2021. PeV-A3 accounted for most typed samples in 5/7 study years: 71% in 2015, 75% in 2016, 61% in 2017, 61% in 2019, and 50% in 2020; A5 accounted for 23/31 (74%) of typed samples in 2018 and A1 for 44/52 (85%) in 2021.
Geographic Distribution of Parechovirus Types
Spain (35%) and Denmark (33%) provided the most parechovirus case reports with typing information (Figure 2). All laboratories performing typing reported PeV-A3 cases, the most being from Spain (n = 138), Denmark (72), and Italy/Lombardy (17). PeV-A3 exhibits a biannual cycle; most parechovirus cases reported by Denmark were identified in even years (2016 and 2018), whereas most cases reported by Spain occurred in uneven years (2015, 2017, and 2019). Denmark and the Netherlands reported the most PeV-A1 and A6 cases; the Netherlands (35.3%, n = 6), Austria (29.4%, n = 5), and Spain (17.6%, n = 3) reported the most A5 cases. Spain reported 8/13 (62%) A4 cases and Poland reported 1 A14 case.
Sample Types
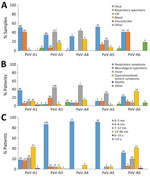
Sample type information was available for 1,294 positive samples from 13 laboratories. Fecal (n = 447; 35%), CSF (391; 30%), and respiratory (259; 20%) specimens were the sample types most often collected for parechovirus testing; in some cases patients might have provided >1 sample type for testing. CSF was the most common specimen type collected in Austria, Luxemburg, Spain, England, and Scotland; feces in Denmark, Ireland, and the Netherlands; and respiratory specimens in Italy/Lombardy and Slovenia.
From the 136 successfully typed CSF samples, PeV-A3 (40%), A4 (44%), and A5 (22.7%) were the only types reported, whereas PeV-A1 (50%), A6 (41%), and A5 (52%) were identified from 208 fecal samples. From the 90 respiratory samples typed, PeV-A1 (61%) was the most commonly reported, followed by A3 (20%), A6 (12%), and A5 (7%); no type A4 was reported in respiratory samples.
Demographic Information and Clinical Manifestations
Demographic information was available for 1,299 and clinical information for 1,269 parechovirus case-patients reported from 14 laboratories in 11 countries. Male patients (61%, n = 763) and infants <1 year of age (76%, n = 987) accounted for most reported cases; infants <3 months of age accounted for 777 (60%) of reported cases. Symptoms were reported for 1,232/1,479 (83%) cases; fever (23%, n = 305) and neurologic signs (21%, n = 280) were the most common, followed by respiratory symptoms (13%, n = 170). Among patients with less common signs and symptoms, 45 (3.4%) children manifested sepsis, 2 were diagnosed with cardiomyopathy, and 1 with hepatitis. Three children diagnosed with PeV-A1 infection in the Netherlands in 2017 died, but it is unknown whether death was related to parechovirus infection.
Information on age groups and symptoms were available for 509/518 (98%) successfully typed cases. The most-reported symptom was fever in children infected with PeV-A3 (44%), A4 (50%), and A5 (30%); among children infected with PeV-A6, gastrointestinal (35%) and respiratory (25%) symptoms were the most commonly reported. Respiratory symptoms (37%) were also common among children infected with PeV-A1 (Figure 3). Most children infected with PeV-A3 (87%), A4 (92%), and A5 (91%) were <3 months of age, whereas >82% of children infected with PeV-A1 were >3 months of age (p<0.0001). Parechovirus infections were rare (n = 68) in children and persons >15 years of age; in that age range, only 1/68 viruses was successful typed and identified as PeV-A3. All detected parechovirus types were associated with neurologic symptoms, of which 72% were typed as PeV-A3, followed by A1 (11%), A5 (7%), A6 (6%), and A4 (1%). The sole PeV-A14 case was detected in a fecal specimen collected from a child with neurologic symptoms from the 6–15-year age group.
Phylogenetic Analysis
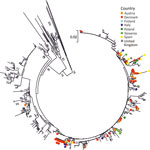
PeV-A3 was the type most frequently reported by participating laboratories. We performed phylogenetic analysis of 106 available study sequences in the VP3/VP1 junction region to compare relationships between potential country- or region-specific groups of strains and available previously published PeV-A3 variants (Figure 4; Appendix Figure 2). Whereas the resolution of the tree was limited by the relatively short length of sequences analyzed (256 bp), variants from different study regions showed some evidence of clustering, possibly representing local geographic spread (e.g., in Denmark), although there was no evidence for specific variants circulating exclusively in just 1 or a few countries. Numerous separate older lineages of PeV-A3 circulating during 2010–2014 or earlier have largely become extinct (Appendix Figure 2).
We report the laboratory capacity, type-related temporal dynamics, epidemiology, and clinical manifestations of parechovirus infections reported from 21 laboratories in 18 countries in Europe over a 7-year study period, 2015–2021. We documented extensive capacity for parechovirus detection in northern, western, and some central European countries participating in our study; no parechovirus testing was reported in Bulgaria, the Czech Republic, the Slovak Republic, Estonia, or Hungary. Those findings were consistent with literature in which limited capacity for parechovirus detection and typing was reported outside western and northern European countries (18–22).
A total of 1,845 parechovirus infections, most identified through NPEV surveillance systems, were reported by 16 laboratories from 13 countries in Europe that participated in the study. Four national laboratories incorporated parechovirus detection into NPEV passive surveillance, collecting data on positive cases from other laboratories that send samples for sequencing after identifying parechovirus. A similar passive surveillance system, in which laboratories report positive NPEV and parechovirus cases to the Centers for Disease Control and Prevention (CDC), was implemented in the United States (23). During 2014–2016 in the United States, ≈100 domestic parechovirus cases were reported to CDC (24); in Europe, 540 cases from Finland, the Netherlands, Spain, and England, were reported over a comparable 3-year timeframe, 2015–2017. Those figures highlight the current volume and likely benefits of the data collected in Europe, along with the potential capacity to implement similar systems in additional countries within and beyond our region.
The capacity for parechovirus testing increased during the study period from ≈9,000 samples tested for parechovirus during 2015–2017 to >14,000 during 2018–2021. Luxemburg, Poland, and Slovenia successfully introduced parechovirus testing in 2017, but some 2018–2021 increases in detection capacity attributable to new data sources were likely offset by several laboratories substantially reducing diagnostic and surveillance testing capacity for pathogens not related to SARS-CoV-2 during the COVID-19 pandemic. The overall detection rate of 1.3% (309/22,928) was lower than previously observed rates of 2%–3% in Denmark (21) and 13% in Northern Ireland (22). However, it is difficult to compare results from our study with results from studies that focused mainly on select populations, such as children and infants needing intensive care unit admission (4).
Besides countries with passive surveillance, laboratories in 2 countries introduced parechovirus testing for respiratory samples collected during ILI surveillance; because samples were implicitly collected from persons with respiratory symptoms only, persons with other parechovirus symptoms would not have been captured through those means. Although ILI surveillance covered all age groups, young infants were likely underrepresented because only 12/130 parechovirus-positive samples were collected from children <3 months of age, which might explain why most of the parechovirus infections captured through ILI surveillance were identified as PeV-A1, a type uncommon among the youngest infants. Based on this finding, ILI surveillance is less likely to capture PeV-A3 infections in children, especially those <3 months of age, because A3 infection manifests with only respiratory symptoms very rarely. Using only ILI surveillance therefore might not be the best option for identifying parechovirus (25).
Twelve laboratories that reported typing capacity successfully sequenced ≈45% of their positive samples, so 28% of total parechovirus-positive samples reported in this study were typed. PeV-A3, the most common type identified in this study, was mostly associated with neurologic infections in infants <3 months of age. The association of PeV-A3 with severe disease, especially in young children, has been well documented elsewhere (4,5,8,26–29). Our study confirmed both PeV-A3 detection in infants <3 months of age (77% of all typed cases were from this age group) and its severity of infection (73% of infants <3 months of age manifested neurologic signs). Detection of PeV-A3 in sterile samples, such as CSF and blood, confirms its likely systemic nature, which often leads to severe infection. Most PeV-3 cases were originally identified in even-numbered years (2008, 2010, 2012, 2014, and 2016) in northern Europe, the United States, and Australia (18,19,30,31). That biannual seasonal pattern was observed for PeV-A3 in Denmark in spring/summer of 2016–2018, but A3 infections appeared to follow a different 2-year cycle in Spain, with peaks in 2017 and 2019. PeV-A1, on the other hand, appeared to follow an annual cycle peaking later each year. Phylogenetic analysis revealed no notable geographic or seasonal clustering of PeV-A3.
A 2022 increase in PeV-A3 infections affecting newborns and young infants and often resulting in severe outcomes was noted in the United States using data from its passive surveillance system (32–34). Those data were used to encourage clinicians to consider parechovirus as a differential diagnosis in cases of fever, sepsis-like syndrome, seizures, or meningitis without another known cause (32,33). Although our findings demonstrate that passive parechovirus surveillance and diagnostic capacities are already available in Europe, no upsurge in recorded parechovirus infections has been noted to date. In future, better harmonization of data collection could be used to monitor the spread of parechovirus infections across Europe, complement early warning systems, and provide the bases for public health recommendations during upsurges.
Despite ongoing collection and testing of samples during the COVID-19 pandemic, parechovirus detection frequencies for A3–A6 declined dramatically in 2020–2021 during periods of lockdown, comparable to previously documented decreases observed for enteroviruses, such as enterovirus D68 (35). An upsurge in PeV-A1 but not in other types in autumn 2021 mirrored the timing of the reappearance of enterovirus D68 and coincided with the end of COVID-19 lockdown restrictions and increased testing of respiratory samples (35). This suggests that PeV-A1 more likely spreads through respiratory routes than other parechovirus types.
In terms of clinical associations, our large-scale description of cases provides evidence for differentiating disease patterns between parechovirus types. A4 and A5 infections were detected largely in infants <3 months of age and more often in sterile samples, such as CSF and blood (Figure 3), both features comparable to previously described epidemiologic and clinical properties of PeV-A3 (11,36,37). Strikingly, parechovirus types A4 and A5 were also primarily detected in children <3 months of age, but PeV-A1 and A6 infections occurred mainly in children 1–5 years of age.
Fever and a higher frequency of neurologic symptoms were associated with higher percentages of PeV-A3 (44%), A4 (50%), and A5 (30%) than A1 or A6 cases. Further patient characterization is required to evaluate whether PeV-A4 and A5 might be more likely to cause neurologic diseases resembling those from PeV-A3 (10,31–33). Although clinical profiles in our study indicate similar neurologic manifestations for PeV-A3 and A4, another study reported that only 9% of A4 infections resulted in neurologic symptoms, much lower than for A3 (91%) (10). It should be noted that almost all PeV-A5 infections in our study were reported by Austria, the Netherlands, and Spain in 2018, and more recently by Italy/Lombardy. Therefore, clinical attributes related to neurologic effects might reflect biologic characteristics of circulating strains rather than differences in parechovirus type.
Collected data were reported as aggregated information, limiting the possibility of calculating risk ratios for associations between specific parechovirus types and clinical symptoms. In addition, each country used different case definitions and criteria for collecting and testing samples. Those limitations should inform interpretation of results and their use as baseline information for future systematic approaches.
In conclusion, we demonstrate that multiple laboratories located in 13 countries in Europe have been collecting and analyzing data on parechovirus infections, including demographic information, clinical features, specimen types, and type sequences. Results of investigating parechovirus epidemiology and collecting and analyzing an increasing amount of data suggest that this virus causes severe infections, especially in very young children. Those findings highlight the need to expand parechovirus diagnostics and typing beyond current participating laboratories and share protocols to develop and initiate more efficient systematic approaches for identifying parechovirus-positive cases in Europe. Future approaches should also include a wider spectrum of age-groups and clinical symptoms. Integrating parechovirus with NPEV surveillance would enable better characterization of parechovirus types and seasonality across and beyond Europe and support outbreak detection to improve clinical and public health awareness and provide resources to limit the spread of parechovirus in Europe.
Dr. Bubba is a public health microbiologist who is a consultant for the World Health Organization and collaborating with ENPEN. Her research interests include syndromic-sentinel surveillance, wastewater surveillance, molecular epidemiology, phylogeny, and laboratory capacity building on both viral and bacterial infections.
Acknowledgments
We thank the European Centre of Control and Diseases training office for the opportunity to conduct this project as part of the European Public Health Microbiology fellowship. We also thank Sandro Binda and Cristina Galli for support and help with this study; Federica Giardina for sequencing support; Spanish Study Group for Pediatric Enterovirus and Parechovirus Infections; and G. Megias, J. Valencia, M. Aranzamendi, A. Gutierrez-Arroyo, C. Muñoz-Almagro, C. Launes, A. Moreno Docón, N. Rabella, C. Berengua, A. Navascués, S. Rey Cao, and M.C. Nieto.
A.P. and B.F. were supported by EU funding within the NextGeneration EU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT); M.W. and A.K. were supported by the National Institute of Public Health NIH–NRI (BW-1/2023).
ENPEN working group: Monika Redlberger–Fritz, Stephan Aberle (Medical University Vienna Center for Virology, Vienna, Austria); Lubomira Nikolaeva-Glomb (National Center of Infectious and Parasitic Diseases, Sofia, Bulgaria); Petra Rainetova (National Institute Public Health, Prague, Czech Republic); Sofie E. Midgley, Kristina Træholt Franck (Statens Serum Institut, Copenhagen, Denmark); Annemarjut J. Jääskeläinen (Helsinki University Hospital HUS Diagnostic Center, Helsinki, Finland); Teemu Smura (University of Helsinki, Helsinki, Finland); Agnes Farkas (National Public Health Center, Budapest, Hungary); Ursula Morley, Cillian De Gascun (University College National Virus Reference Laboratory, Dublin, Ireland); Elena Pariani, Laura Pellegrinelli (University of Milan Department of Biomedical Sciences for Health, Milan, Italy); Fausto Baldanti, Antonio Piralla (Università degli Studi di Pavia Department of Clinical Surgical Diagnostic and Paediatric Sciences, Pavia, Italy); Massimo Oggioni (Azienda Socio-Sanitaria Territoriale della Brianza Microbiologia e Virologia Clinica, Vimercate, Italy); Sara Uceda Renteria (Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico Virology Unit, Milan, Italy); Trung Nguyen, Sibel Berger (Laboratoire national de Santé, Dudelange, Luxembourg); Kimberley Benschop, (National Institute for Public Health and the Environment [RIVM], Bilthoven, the Netherlands), Katja Wolthers (OrganoVIR Labs, Department of Medical Microbiology, Amsterdam UMC, Amsterdam, the Netherlands); Svein Nordbo, Susanne Gjeruldsen Dudman (St. Olavs University Hospital Department of Medical Microbiology, Trondheim, Norway); Magdalena Wieczorek, Arleta Krzysztoszek (National Institute of Public Health NIH–NRI Department of Virology, Warsaw, Poland); Katarína Pastuchová (Public Health Authority of the Slovak Republic, Bratislava, Slovak Republic); Nataša Berginc (National Laboratory for Health, Environment and Food, Laboratory for Public Health Virology, Maribor, Slovenia); Mario Poljak, Maja M. Lunar (University of Ljubljana Institute of Microbiology and Immunology, Ljubljana, Slovenia); Maria Cabrerizo, M. Dolores Fernandez-Garcia (National Centre for Microbiology Instituto de Salud Carlos III and Center for Biomedical Network Research on Epidemiology and Public Health [CIBERESP], Madrid, Spain); Cristina Calvo (La Paz University Hospital Department of Paediatric Infectious Diseases, and Translational Research Network in Paediatric Infectious Diseases [IDIPAZ], Madrid, Spain) Cristina Celma, Yasmin Mohammadi (UK Health Security Agency, London, UK); Kate Templeton (University of Edinburgh, Edinburgh, Scotland, UK).
References
- Harvala H, Wolthers KC, Simmonds P. Parechoviruses in children: understanding a new infection. Curr Opin Infect Dis. 2010;23:224–30. DOIPubMedGoogle Scholar
- Wang CYT, Ware RS, Lambert SB, Mhango LP, Tozer S, Day R, et al. Parechovirus A infections in healthy Australian children during the first 2 years of life: a community-based longitudinal birth cohort study. Clin Infect Dis. 2020;71:116–27. DOIPubMedGoogle Scholar
- Olijve L, Jennings L, Walls T. Human parechovirus: an increasingly recognized cause of sepsis-like illness in young infants. Clin Microbiol Rev. 2017;31:e00047–17.DOIPubMedGoogle Scholar
- Harvala H, Robertson I, Chieochansin T, McWilliam Leitch EC, Templeton K, Simmonds P. Specific association of human parechovirus type 3 with sepsis and fever in young infants, as identified by direct typing of cerebrospinal fluid samples. J Infect Dis. 2009;199:1753–60. DOIPubMedGoogle Scholar
- Wolthers KC, Benschop KS, Schinkel J, Molenkamp R, Bergevoet RM, Spijkerman IJ, et al. Human parechoviruses as an important viral cause of sepsislike illness and meningitis in young children. Clin Infect Dis. 2008;47:358–63. DOIPubMedGoogle Scholar
- Harvala H, Calvert J, Van Nguyen D, Clasper L, Gadsby N, Molyneaux P, et al. Comparison of diagnostic clinical samples and environmental sampling for enterovirus and parechovirus surveillance in Scotland, 2010 to 2012. Euro Surveill. 2014;19:20772. DOIPubMedGoogle Scholar
- Zell R, Delwart E, Gorbalenya AE, Hovi T, King AMQ, Knowles NJ, et al.; Ictv Report Consortium. ICTV virus taxonomy profile: Picornaviridae. J Gen Virol. 2017;98:2421–2. DOIPubMedGoogle Scholar
- Harvala H, McLeish N, Kondracka J, McIntyre CL, McWilliam Leitch EC, Templeton K, et al. Comparison of human parechovirus and enterovirus detection frequencies in cerebrospinal fluid samples collected over a 5-year period in edinburgh: HPeV type 3 identified as the most common picornavirus type. J Med Virol. 2011;83:889–96. DOIPubMedGoogle Scholar
- Harvala H, Robertson I, McWilliam Leitch EC, Benschop K, Wolthers KC, Templeton K, et al. Epidemiology and clinical associations of human parechovirus respiratory infections. J Clin Microbiol. 2008;46:3446–53. DOIPubMedGoogle Scholar
- Sasidharan A, Harrison CJ, Banerjee D, Selvarangan R. Emergence of parechovirus A4 central nervous system infections among infants in Kansas City, Missouri, USA. J Clin Microbiol. 2019;57:e01698–18. DOIPubMedGoogle Scholar
- Chamings A, Liew KC, Reid E, Athan E, Raditsis A, Vuillermin P, et al. An emerging human parechovirus type 5 causing sepsis-like illness in infants in Australia. Viruses. 2019;11:913. DOIPubMedGoogle Scholar
- Sridhar A, Karelehto E, Brouwer L, Pajkrt D, Wolthers KC. Parechovirus A pathogenesis and the enigma of genotype A-3. Viruses. 2019;11:1062. DOIPubMedGoogle Scholar
- EUSurvey. HPeV circulation in EU/EEA & UK, 2015–2021 [cited 2022 Mar 10]. https://ec.europa.eu/eusurvey/runner/HPeV_circulation_in_EU-EEA_UK_2015-2021
- Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26:3661–75. DOIPubMedGoogle Scholar
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7. DOIPubMedGoogle Scholar
- Simmonds P. SSE: a nucleotide and amino acid sequence analysis platform. BMC Res Notes. 2012;5:50. DOIPubMedGoogle Scholar
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. DOIPubMedGoogle Scholar
- Marchand S, Launay E, Schuffenecker I, Gras-Le Guen C, Imbert-Marcille BM, Coste-Burel M. Severity of parechovirus infections in infants under 3 months of age and comparison with enterovirus infections: A French retrospective study. Arch Pediatr. 2021;28:291–5. DOIPubMedGoogle Scholar
- Elling R, Böttcher S, du Bois F, Müller A, Prifert C, Weissbrich B, et al. Epidemiology of human parechovirus type 3 upsurge in 2 hospitals, Freiburg, Germany, 2018. Emerg Infect Dis. 2019;25:1384–8. DOIPubMedGoogle Scholar
- Linhares MI, Brett A, Correia L, Pereira H, Correia C, Oleastro M, et al. Parechovirus genotype 3 outbreak among young infants in Portugal. Acta Med Port. 2021;34:664–8. DOIPubMedGoogle Scholar
- Fischer TK, Midgley S, Dalgaard C, Nielsen AY. Human parechovirus infection, Denmark. Emerg Infect Dis. 2014;20:83–7. DOIPubMedGoogle Scholar
- Davis J, Fairley D, Christie S, Coyle P, Tubman R, Shields MD. Human parechovirus infection in neonatal intensive care. Pediatr Infect Dis J. 2015;34:121–4. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. National Enterovirus Surveillance System (NESS): surveillance data [cited 2023 Aug 28]. https://www.cdc.gov/surveillance/ness/surv-data.html
- Abedi GR, Watson JT, Nix WA, Oberste MS, Gerber SI. Enterovirus and parechovirus surveillance—United States, 2014–2016. MMWR Morb Mortal Wkly Rep. 2018;67:515–8. DOIPubMedGoogle Scholar
- Kadambari S, Harvala H, Simmonds P, Pollard AJ, Sadarangani M. Strategies to improve detection and management of human parechovirus infection in young infants. Lancet Infect Dis. 2019;19:e51–8. DOIPubMedGoogle Scholar
- Benschop KS, Schinkel J, Minnaar RP, Pajkrt D, Spanjerberg L, Kraakman HC, et al. Human parechovirus infections in Dutch children and the association between serotype and disease severity. Clin Infect Dis. 2006;42:204–10. DOIPubMedGoogle Scholar
- Piralla A, Furione M, Rovida F, Marchi A, Stronati M, Gerna G, et al. Human parechovirus infections in patients admitted to hospital in Northern Italy, 2008-2010. J Med Virol. 2012;84:686–90. DOIPubMedGoogle Scholar
- Harvala H, Griffiths M, Solomon T, Simmonds P. Distinct systemic and central nervous system disease patterns in enterovirus and parechovirus infected children. J Infect. 2014;69:69–74. DOIPubMedGoogle Scholar
- Esposito S, Rahamat-Langendoen J, Ascolese B, Senatore L, Castellazzi L, Niesters HG. Pediatric parechovirus infections. J Clin Virol. 2014;60:84–9. DOIPubMedGoogle Scholar
- van der Sanden S, de Bruin E, Vennema H, Swanink C, Koopmans M, van der Avoort H. Prevalence of human parechovirus in the Netherlands in 2000 to 2007. J Clin Microbiol. 2008;46:2884–9. DOIPubMedGoogle Scholar
- Nelson TM, Vuillermin P, Hodge J, Druce J, Williams DT, Jasrotia R, et al. An outbreak of severe infections among Australian infants caused by a novel recombinant strain of human parechovirus type 3. Sci Rep. 2017;7:44423. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Emergency preparedness and response: recent reports of human parechovirus (PeV) in the United States—2022 [cited 2022 Jul 11]. https://emergency.cdc.gov/han/2022/han00469.asp
- South Carolina Department of Health and Environmental Control; South Carolina Health Alert Network. CDC health advisory: recent reports of human parechovirus (PeV) in the United States—2022 [cited 2022 Jul 13]. https://scdhec.gov/sites/default/files/media/document/10523-CHA-07-13-2022-PeV.pdf
- Victoria Department of Health. Human parechovirus type 3 in Victoria [cited 2022 Nov 7]. https://www.health.vic.gov.au/health-advisories/human-parechovirus-type-3-in-victoria
- Benschop KS, Albert J, Anton A, Andrés C, Aranzamendi M, Armannsdóttir B, et al. Re-emergence of enterovirus D68 in Europe after easing the COVID-19 lockdown, September 2021. Euro Surveill. 2021;26:
2100998 . DOIPubMedGoogle Scholar - Kolehmainen P, Jääskeläinen A, Blomqvist S, Kallio-Kokko H, Nuolivirta K, Helminen M, et al. Human parechovirus type 3 and 4 associated with severe infections in young children. Pediatr Infect Dis J. 2014;33:1109–13. DOIPubMedGoogle Scholar
- Piralla A, Perniciaro S, Ossola S, Giardina F, De Carli A, Bossi A, et al. Human parechovirus type 5 neurological infection in a neonate with a favourable outcome: A case report. Int J Infect Dis. 2019;89:175–8. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: January 17, 2024
Table of Contents – Volume 30, Number 2—February 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Laura Bubba, European Non-polio Enterovirus Network (ENPEN), Milan 20133, Italy
Top