Volume 30, Number 3—March 2024
Research
Microsporidia (Encephalitozoon cuniculi) in Patients with Degenerative Hip and Knee Disease, Czech Republic
Cite This Article
Citation for Media
Abstract
Total joint arthroplasty is a commonly used surgical procedure in orthopedics. Revision surgeries are required in >10% of patients mainly because of prosthetic joint infection caused by bacteria or aseptic implant loosening caused by chronic inflammation. Encephalitozoon cuniculi is a microsporidium, an obligate intracellular parasite, capable of exploiting migrating proinflammatory immune cells for dissemination within the host. We used molecular detection methods to evaluate the incidence of E. cuniculi among patients who had total hip or knee arthroplasty revision. Out of 49 patients, E. cuniculi genotypes I, II, or III were confirmed in joint samples from 3 men and 2 women who had implant loosening. Understanding the risks associated with the presence of microsporidia in periprosthetic joint infections is essential for proper management of arthroplasty. Furthermore, E. cuniculi should be considered a potential contributing cause of joint inflammation and arthrosis.
Microsporidia are a group of obligate intracellular parasites comprising ≈1,300 species within >200 genera (1). Microsporidia are considered to be closely related to fungi (2,3) and infect a broad range of invertebrates and vertebrates, from protists to humans (4). Because of improved detection methods and greater awareness, microsporidia have been detected in a broad range of human populations, including children, travelers, elderly persons, and organ transplant recipients (5). Persons with high exposure to animals and contaminated soil and water are considered at risk for microsporidiosis (6). Of the several species of microsporidia that infect humans, Encephalitozoon cuniculi is the most common (7). Four genotypes of E. cuniculi have been identified on the basis of variable repeats in the rRNA internal transcribed spacer; however, human infections are mostly associated with genotypes I and II (8).
The digestive tract is an entrance point for microsporidia and subsequent spreading of infection occurs in all parts of the intestine. Within weeks, infection spreads to other tissues and organs, most commonly the kidney, liver, spleen, lung, and brain, depending on the species-specific interaction with the host (8). However, the unique mechanism of host cell invasion involving a highly specialized structure, the 10–50 μm long polar filament, enables only limited spread over short distances within the host. Therefore, the dissemination rate suggests the possible engagement of macrophages or other immune cells involved in inflammatory responses, which can serve as vehicles transporting microsporidia to foci outside of the intestine (9,10). Microsporidia are often overlooked in clinical samples because of problematic diagnoses, increasing the likelihood of hidden infections that can cause extensive tissue damage and various nonspecific pathologies and that often go without effective treatment (11).
Total joint arthroplasty is one of the most common surgical procedures in orthopedics to replace joints in patients with degenerative diseases (12). Revision surgeries are required in >10% of those patients because of implant failure caused mainly by prosthetic joint infection and aseptic implant loosening from inflammation (13,14). Whereas prosthetic joint infection is caused by bacterial infection (e.g., Staphylococcus aureus, Streptococcus spp., and Enterococcus faecalis) and pathologic growth around the prosthetic joint (15), aseptic implant loosening results from chronic inflammation caused by activation of resident immune cells in contact with implant wear debris or allergic reactions to metal ions derived from implant materials (16). However, the classification of aseptic implant loosening might be misleading because other pathogens are often overlooked, and the condition is potentially mislabeled as aseptic (17).
E. cuniculi is considered a cause of osteolysis in hip periprosthetic tissue (17), and connections between proinflammatory immune responses and concentration of E. cuniculi in inflammatory foci have been reported (9,10). Understanding the risks for microsporidiosis within periprosthetic joints is essential for proper arthroplasty management. We evaluated the incidence of generally neglected microsporidia among patients who had total hip or knee arthroplasty revision.
Patients
We investigated samples obtained from immunocompetent patients who were hospitalized or who visited the orthopedic clinic at Bulovka Hospital (Prague, Czech Republic) during May 2020–September 2021. The first group of patients had undergone a hip puncture/total hip revision arthroplasty, and the second group had undergone a knee puncture/knee revision arthroplasty (in 1 case, the patient only underwent a knee arthroscopy). We assigned patients to 3 diagnostic groups according to microbiologic cultures and criteria of the Infectious Diseases Society of America or the Musculoskeletal Infection Society for periprosthetic joint infection according to the judgement of the treating physician: periprosthetic joint infection, aseptic implant loosening (aseptic loosening was diagnosed when signs of implant loosening were present, but infection was not the cause), and other diagnosis (patients who did not fit into the first 2 groups) (18,19).
Sample Collection
Fragments of periprosthetic hip and knee tissues and joint fluids were collected intraoperatively; joint aspirates were collected during knee or hip punctures. Samples for microbiologic culture (i.e., samples of joint tissues, joint fluids, and surgical swabs from the endoprosthesis, tissues, or joints) were gathered intraoperatively. The number of samples collected was at the discretion of the orthopedic surgeon. Surgical swabs or other samples with insufficient volumes were excluded from the study. All samples were collected under sterile conditions. Each sample was placed in a separate sterile container and delivered at room temperature (20°–25°C) to the Department of Clinical Microbiology at Bulovka Hospital. Samples collected outside of laboratory working hours were maintained at room temperature (20°–25°C) overnight and then processed.
Samples were processed in a laminar flow cabinet for microbiologic culture; aliquots were stored without preservatives at −20°C for further molecular investigation and sent to the Biology Centre of the Czech Academy of Sciences for microsporidia screening. For Neisseria identification, we inoculated samples onto GO blood agar (LabMediaServis s.r.o., https://www.labmediaservis.cz) and 5% sheep blood agar, and incubated at 37°C in 5% CO2 for 24 h for GO blood agar and 48 h for 5% sheep blood agar. We cultured samples on Endo agar, in liver broth, and on sheep blood agar (containing 10% NaCl) at 37°C in an aerobic atmosphere for 24 h and 48 h (blood agar). After 24 h, we subcultured the liver broth on 5% sheep blood agar in a 5% CO2 atmosphere and Endo agar in an aerobic atmosphere for another 24 h. We examined cultures for bacterial growth after 24 h and 48 h (GO blood agar). If no growth occurred, we incubated the 5% sheep blood agar and GO blood agar cultures for 7 d. For anaerobic cultures, we inoculated patient samples onto Schaedler agar and in thioglycolate broth and cultured in an anaerobic atmosphere for 48 h and a total of 7 d. Depending on the microbiologist’s decision, we subcultured the thioglycolate broth cultures onto Schaedler agar. We identified all bacteria by using standard laboratory procedures, including biochemical testing, by using the BD Phoenix system (Becton Dickinson, https://www.bd.com), and, in the case of Salmonella Enteritidis, by serotyping. We performed antimicrobial drug susceptibility testing by using European Committee on Antimicrobial Susceptibility Testing methodology (https://www.eucast.org). We prepared fungal cultures on Sabouraud agar only when requested by the orthopedic surgeon; those plates were incubated aerobically at 37°C for 48 h, examined, and then cultured for a total of 7 d.
DNA Isolation
We used aliquots of tissue and primary materials from joint aspirates and fluids from each patient for DNA isolation. We homogenized a total of 200 mg of tissue or aspirate sediment by using bead disruption on a FastPrep-24 instrument (MP Biomedicals, https://www.mpbio.com) at a speed of 5.5 m/s for 1 min. We extracted total DNA by using the DNeasy Blood and Tissue Kit (QIAGEN, https://www.qiagen.com) according to the manufacturer’s instructions. We included an extraction negative control to each DNA extraction series to ensure the absence of contamination in reagents, consumables, and the environment. We stored extracted DNA at −20°C until PCR amplification. We isolated control DNA from purified E. intestinalis spores by using the same methods.
Molecular Examination
We amplified a partial sequence of the 16S rRNA gene that included the entire internal transcribed spacer by using nested PCR protocols with microsporidia-specific primers (9). We used DNA obtained from E. intestinalis spores as a positive PCR control and ultrapure water (without template) as a negative control in each PCR run. We evaluated the PCR products by gel electrophoresis.
We processed DNA from microsporidia PCR-positive samples by using a real-time quantitative PCR protocol that amplified a 268-bp region of the E. cuniculi 16S rRNA gene (9). We used negative controls comprising unspiked specimens and diluent blanks for each PCR. We determined positive results according to mathematical algorithms included with the LightCycler System (Roche, https://www.roche.com); results were positive when the cycle threshold was <43. We calculated the total number of spores in 1 g of sample according to a standard curve derived from spore DNA that was serially diluted in water; dilutions ranged from 1 to 1 × 108 (R2 = 0.9903).
Phylogenetic Analyses
We purified PCR amplicons by using the QIAquick Gel Extraction Kit (QIAGEN), and sequencing was performed in both directions at SeqMe (https://www.seqme.eu). Amplification and sequencing of each positive sample was repeated 3 times.
We manually edited the nucleotide sequences by using ChromasPro 2.1.4 (Technelysium, https://www.technelysium.com.au) and aligned the sequences with references from GenBank by using MAFFT version 7 (http://mafft.cbrc.jp). We performed phylogenetic analysis by using the maximum-likelihood method and evolutionary models selected by MEGA X software (MEGA, https://www.megasoftware.net). We inferred the evolutionary history for partial sequences of the 16S rRNA gene, the entire internal transcribed spacer region, and a partial sequence of the 5.8S rRNA gene by using neighbor-joining analyses and computed relationships between sequences by using the Tamura 3-parameter method, gamma distribution, and parametric bootstrap analysis of 1,000 replicates in MEGA X software.
Microscopic Examination
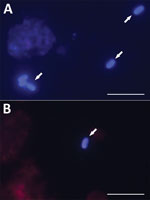
We examined microsporidia PCR-positive samples microscopically. We prepared slides by mechanically homogenizing tissue samples with a mortar and pestle and centrifuged aspirates at 13,000 × g for 10 min; we stained aspirate sediments and homogenized tissues with Calcofluor M2R (Sigma Aldrich, https://www.sigmaaldrich.com) (17).
Ethics Statement
We analyzed existing specimens beyond routine microbiologic screening, focusing on verifying the association between inflammatory disease and the presence of microsporidia in inflammatory foci. Because the study was performed by using samples with no human intervention arm, patient consent was not required.
We screened a total of 94 samples from 49 patients who were 41–96 (median 71) years of age for microsporidia infection in tissues surrounding the operated hip and knee joints. The mean age was 71 (SD+9.3; range 41–84) among hip replacement patients and 70 (SD+9.0; range 62–96) years among knee replacement patients. The male to female ratio was 12 (36%) to 21 (64%) in the hip replacement group and 9 (56%) to 7 (44%) in knee replacement group. Most patients had prosthetic joint infections; only 3 patients had other diagnoses, and the remaining patients had aseptic implant loosening (Table 1). Laboratory examinations showed physiologic indicators were within reference ranges for all patients. Patients did not undergo immunosuppressive treatment during the study period.
Among screened patients, 16 underwent knee arthroplasty providing 36 samples, and 33 underwent hip arthroplasty providing 58 samples (Table 1). The number of samples obtained from patients was 1–7; multiple samples mostly represented more sample types (Figure 1). Most (n = 28) patients underwent primary revision, then secondary and further revisions (9 each); 3 patients underwent repeated surgery: primary/secondary revision (patient no. 24) and primary/third and further revision (patient nos. 9 and 19) (Figure 1).
Of the 94 samples examined, most (61) were microbiologically sterile, whereas 12 samples were positive for S. aureus (5 were methicillin resistant), 6 were positive for Escherichia coli, 3 were positive for E. faecalis, 3 were positive for Salmonella Enteritidis, 2 were positive for Staphylococcus epidermidis, and 2 were positive for group G beta-hemolytic Streptococcus. Streptococcus agalactiae, Corynebacterium tuberculostearicum, Pseudomonas aeruginosa, or Enterococcus faecium were detected in the remaining samples.
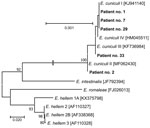
Encephalitozoon-specific DNA was confirmed in samples from 3 men and 2 women who were 63–78 years of age. Phylogenetic analyses revealed E. cuniculi genotypes I, II, and III. The 5 sequences obtained in this study were 100% identical to GenBank sequences for E. cuniculi genotype I (accession no. KJ941140), II (accession no. MF062430), and III (accession no. KF736984) (Figure 2).
We detected microsporidia in knee or hip aspirates obtained during ambulatory puncture and joint fluids and tissues recovered intraoperatively for all 5 Encephalitozoon-positive patients (Table 2). Of those 5 patients, 3 had periprosthetic joint infection, and 2 had aseptic implant loosening. E. cuniculi genotype I was most often detected, in 8 knee and hip samples from 3 patients; the number of spores ranged from 12 to 5,600 per gram of sample. We detected Encephalitozoon cuniculi genotype II in a hip sample (260 spores/g sample) from 1 patient, and genotype III in a knee sample (6.9 spores/g sample) from 1 other patient (Table 2). Microscopic analysis of Calcofluor M2R–stained smears confirmed the presence of spores (2–5 spores per slide) in tissue samples obtained from patient nos. 2 and 29 who tested positive for Encephalitozoon DNA (Figure 3). Samples from the other 3 patients were microscopically negative for spores. Microbiologic tests showed bacterial infections within the tissues of 3 patients: group G beta-hemolytic Streptococcus in the knee of patient no. 1, E. faecalis in the knee of patient no. 29, and methicillin-resistant S. aureus in the hip of patient no. 2; the other 2 patients were clinically classified as aseptic (Table 2).
Primary hip and knee arthroplasty ranks among the top 5 most common procedures performed and among the top 5 fastest growing procedures each year across all surgical disciplines (20). Total joint replacement improves function, reduces pain, and improves quality of life for patients, and is cost-effective (21,22). Despite the high success rate of modern total joint arthroplasty (23) and technologic advances designed to extend the lifetime of primary implants (24–26), modern implant bearings and well-fixed components have a finite lifespan (27). Total joint replacements because of osteoarthritis require a revision procedure in 10% of patients, ≈4% within 10 years of initial surgery (13,14). Risk for revision increases in younger, more active patients and in those who have a higher body mass index (28). The most common reasons for revision surgery are infection, fracture around the implant, and loosening of the implant, which can occur soon after joint replacement or after decades of good function (29).
Prosthetic joint infection was detected in 34 (69.3%) of 49 patients we screened. Gram-positive cocci, such as S. aureus, coagulase-negative staphylococci, and E. faecalis are the major prosthetic joint infection-related microorganisms, after which Gram-negative bacilli are common (30–32); however, other pathogens are often overlooked, leading to an aseptic joint diagnosis. Microsporidia are often overlooked, fungus-related, obligate intracellular parasites occurring worldwide and infecting various vertebrate and invertebrate hosts, including humans (33,34); 17 species have been reported in humans, causing more severe symptoms in immunocompromised persons than in immunocompetent counterparts (35,36). E. cuniculi was the first microsporidium identified in mammals and the best-studied, forming the foundation of knowledge about microsporidia. E. cuniculi is typically described as a chronic, slow-acting pathogen and, thus, is considered less virulent than other pathogen groups; however, it can multiply successfully and extensively without any obvious signs of infection in immunocompetent hosts (37–39). E. cuniculi infects a wide spectrum of host cells, including epithelial cells, vascular endothelial cells, kidney tubule cells, and can be found in most tissues, having a propensity toward brain and kidneys (40). E. cuniculi is responsible for various pathologies depending on the infection site, affecting the nervous system as well as the respiratory and digestive tracts and causing hepatitis, peritonitis, pneumonitis, cystitis, nephritis, and encephalitis (41,42). Most documented cases originated from HIV/AIDS patients and transplant recipients. Whereas infection with E. cuniculi genotype I and II is common, occurrence of genotypes III and IV in humans is rare (43). As researchers and clinicians become more aware of those pathogens and are able to diagnose infections caused by them, new associations between microsporidia parasites and common infections have been reported (17,44). Moreover, E. cuniculi is able to survive and replicate in a variety of immune cells, including resident and migratory macrophages and other phagocytic cells, such as neutrophils, eosinophils, monocytes, and dendritic cells; thus, those immune cells might contribute to dissemination of E. cuniculi throughout the host organism (45,46).
The most common route of microsporidia transmission is the fecal-oral route; spores are passed in the urine or feces of infected persons into the environment and transmitted mostly through contaminated water sources (43). Microsporidia spores have been identified in wastewater, and in surface, irrigation, and drinking water. Moreover, several studies have reported foodborne transmission through fresh produce, such as strawberries, raspberries, lettuce, celery, parsley, and oranges, including orange juice. Recently, E. cuniculi has been reported in milk from dairy cows and goats, and the possibility of E. cuniculi transmission through pasteurized cow’s milk, fermented pork products, and fresh goat cheese has been experimentally documented (43). Furthermore, infection in the respiratory tract suggests airborne transmission by contaminated aerosols (43).
E. cuniculi can survive and persist in immunocompetent hosts, even after chemotherapeutic treatment (47–49), and a latent infection can be activated by inflammation in the host body (9). A role for proinflammatory immune cells in the expansion of E. cuniculi infection in host tissues has been suggested because of the occurrence of microsporidia in inflamed tissues (17) and the targeted migration toward inflammatory foci seen after experimental induction of inflammation (9,10). Thus, the incidence of microsporidia infections might be much higher than previously reported, and microsporidia might represent a neglected etiologic agent for more common diseases, including prosthetic joint infection. We confirmed periprosthetic E. cuniculi infection in 3 patients who had prosthetic joint infection and 2 who had aseptic implant loosening. Moreover, the molecular data were supported by microscopy in 2 patients who had the highest spore loads. The other 3 E. cuniculi PCR-positive patients had negative microscopic results; those results were likely caused by limited sensitivity of microscopy in samples with low spore load rather than laboratory contamination of PCR. Because we obtained uniform results from multiple samples from specific patients by using both PCR and quantitative PCR, it is unlikely that contamination occurred in all samples from a particular patient at the same time and not in other samples. Laboratory contamination was excluded as a possible reason for our results because the samples were taken and PCR was performed under sterile conditions by the same trained personnel, and the PCR diagnostics workspace is structurally divided into separate areas adhering to a one-direction workflow.
Whether microsporidia infection occurred in the affected joint areas before the onset of inflammatory processes or whether they entered the affected areas secondarily through macrophages or other cells involved in inflammation remains unclear. Nevertheless, not only infective agents can induce inflammation. Implant-derived wear particles can also induce host inflammatory responses via opsonization by danger-associated molecular pattern molecules and recognition by Toll-like receptors (50). Therefore, E. cuniculi spores likely were transported to the joints within immune cells associated with proinflammatory immune responses.
In conclusion, E. cuniculi can occupy unusual extraintestinal locations, such as joint fluid or tissue, and should be considered a contributing cause of joint inflammation and arthrosis. However, the role of this pathogen in causing osteolysis and subsequent implant loosening needs to be clarified. The presence of microsporidia spores and DNA in periprosthetic tissue of immunocompetent hosts indicates active infection in those patients and should be considered in the history of the disease. In addition, microsporidia should be considered as a potential cause of periprosthetic osteolysis and implant destabilization after hip replacement.
Dr. Sak is a research scientist at the Biology Centre of the Czech Academy of Sciences. His research interests focus on the detection of parasites, such as microsporidia, and diagnostics, isolation, in vitro cultivation, experimental infections, and morphologic and molecular characterization of parasites.
Acknowledgment
This work was supported by grants from the Grant Agency of the Czech Republic (grant nos. 20-10706S and 23-06571S) and National Science Centre, Poland (grant no. 2020/39/O/NZ6/02313). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Cali A, Becnel JJ, Takvorian PM. Microsporidia. In: Archibald JM, Simpson AGB, Slamovits CH, editors. Handbook of the protists, 2nd edition. Cham (CH): Springer; 2017. p. 1559–618.
- Edlind TD, Li J, Visvesvara GS, Vodkin MH, McLaughlin GL, Katiyar SK. Phylogenetic analysis of beta-tubulin sequences from amitochondrial protozoa. Mol Phylogenet Evol. 1996;5:359–67. DOIPubMedGoogle Scholar
- Keeling PJ, Doolittle WF. Alpha-tubulin from early-diverging eukaryotic lineages and the evolution of the tubulin family. Mol Biol Evol. 1996;13:1297–305. DOIPubMedGoogle Scholar
- Wittner M. Historic perspective on the microsporidia: expanding horizons. In: Wittner M, Weiss LM, editors. The microsporidia and microsporidiosis. Washington DC: American Association of Microbiology; 1999. p. 1–6.
- Didier ES, Didier PJ, Snowden KF, Shadduck JA. Microsporidiosis in mammals. Microbes Infect. 2000;2:709–20. DOIPubMedGoogle Scholar
- Sak B, Kučerová Z, Kváč M, Květoňová D, Rost M, Secor EW. Seropositivity for Enterocytozoon bieneusi, Czech Republic. Emerg Infect Dis. 2010;16:335–7. DOIPubMedGoogle Scholar
- Didier ES. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 2005;94:61–76. DOIPubMedGoogle Scholar
- Didier ES, Weiss LM. Microsporidiosis: not just in AIDS patients. Curr Opin Infect Dis. 2011;24:490–5. DOIPubMedGoogle Scholar
- Brdíčková K, Sak B, Holubová N, Květoňová D, Hlásková L, Kicia M, et al. Encephalitozoon cuniculi genotype II concentrates in inflammation foci. J Inflamm Res. 2020;13:583–93. DOIPubMedGoogle Scholar
- Sak B, Holubová N, Květoňová D, Hlásková L, Tinavská J, Kicia M, et al. Comparison of the concentration of Encephalitozoon cuniculi genotypes I and III in inflammatory foci under experimental conditions. J Inflamm Res. 2022;15:2721–30. DOIPubMedGoogle Scholar
- Lallo MA, da Costa LFV, de Castro JM. Effect of three drugs against Encephalitozoon cuniculi infection in immunosuppressed mice. Antimicrob Agents Chemother. 2013;57:3067–71. DOIPubMedGoogle Scholar
- Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467:2606–12. DOIPubMedGoogle Scholar
- Sloan M, Premkumar A, Sheth NP. Projected volume of primary total joint arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100:1455–60. DOIPubMedGoogle Scholar
- Malchau H, Garellick G, Berry D, Harris WH, Robertson O, Kärrlholm J, et al. Arthroplasty implant registries over the past five decades: Development, current, and future impact. J Orthop Res. 2018;36:2319–30. DOIPubMedGoogle Scholar
- Fernandez-Sampedro M, Salas-Venero C, Fariñas-Álvarez C, Sumillera M, Pérez-Carro L, Fakkas-Fernandez M, et al. 26Postoperative diagnosis and outcome in patients with revision arthroplasty for aseptic loosening. BMC Infect Dis. 2015;15:232. DOIPubMedGoogle Scholar
- Hodges NA, Sussman EM, Stegemann JP. Aseptic and septic prosthetic joint loosening: Impact of biomaterial wear on immune cell function, inflammation, and infection. Biomaterials. 2021;278:
121127 . DOIPubMedGoogle Scholar - Kicia M, Wesolowska M, Kopacz Z, Kvác M, Sak B, Sokulska M, et al. Disseminated infection of Encephalitozoon cuniculi associated with osteolysis of hip periprosthetic tissue. Clin Infect Dis. 2018;67:1228–34. DOIPubMedGoogle Scholar
- Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al.; Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–25. DOIPubMedGoogle Scholar
- Fillingham YA, Della Valle CJ, Suleiman LI, Springer BD, Gehrke T, Bini SA, et al. Definition of successful infection management and guidelines for reporting of outcomes after surgical treatment of periprosthetic joint infection: from the workgroup of the Musculoskeletal Infection Society (MSIS). J Bone Joint Surg Am. 2019;101:
e69 . DOIPubMedGoogle Scholar - Fingar KR, Stocks C, Weiss AJ, Steiner CA. Statistical brief #186. Most frequent operating room procedures performed in U.S. Hospitals, 2003–2012. In Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville (MD): Agency for Healthcare Research and Quality; 2006.
- Price AJ, Longino D, Rees J, Rout R, Pandit H, Javaid K, et al. Are pain and function better measures of outcome than revision rates after TKR in the younger patient? Knee. 2010;17:196–9. DOIPubMedGoogle Scholar
- Dakin H, Gray A, Fitzpatrick R, Maclennan G, Murray D; KAT Trial Group. Rationing of total knee replacement: a cost-effectiveness analysis on a large trial data set. BMJ Open. 2012;2:
e000332 . DOIPubMedGoogle Scholar - Quintana JM, Arostegui I, Escobar A, Azkarate J, Goenaga JI, Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168:1576–84. DOIPubMedGoogle Scholar
- Lim SJ, Jang SP, Kim DW, Moon YW, Park YS. Primary ceramic-on-ceramic total hip arthroplasty using a 32-mm ceramic head with a titanium-alloy sleeve. Clin Orthop Relat Res. 2015;473:3781–7. DOIPubMedGoogle Scholar
- Delaunay CP, Putman S, Puliéro B, Bégin M, Migaud H, Bonnomet F. Cementless total hip arthroplasty with Metasul bearings provides good results in active young patients: a concise followup. Clin Orthop Relat Res. 2016;474:2126–33. DOIPubMedGoogle Scholar
- Sobieraj M, Marwin S. Ultra-high-molecular-weight polyethylene (UHMWPE) in total joint arthroplasty. Bull Hosp Jt Dis (2013). 2018;76:38–46.PubMedGoogle Scholar
- Schwartz AM, Farley KX, Guild GN, Bradbury TL Jr. Projections and epidemiology of revision hip and knee arthroplasty in the United States to 2030. J Arthroplasty. 2020;35(6S):S79–85. DOIPubMedGoogle Scholar
- Bayliss LE, Culliford D, Monk AP, Glyn-Jones S, Prieto-Alhambra D, Judge A, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet. 2017;389:1424–30. DOIPubMedGoogle Scholar
- Rabiu AR, Rasidovic D, Parsons H, Wall PDH, Metcalfe A, Bruce J. Surgical interventions for failed primary knee replacement. Cochrane Database Syst Rev. 2020;2020:
CD013681 . - Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–54. DOIPubMedGoogle Scholar
- Martínez-Pastor JC, Muñoz-Mahamud E, Vilchez F, García-Ramiro S, Bori G, Sierra J, et al. Outcome of acute prosthetic joint infections due to gram-negative bacilli treated with open debridement and retention of the prosthesis. Antimicrob Agents Chemother. 2009;53:4772–7. DOIPubMedGoogle Scholar
- Hsieh PH, Lee MS, Hsu KY, Chang YH, Shih HN, Ueng SW. Gram-negative prosthetic joint infections: risk factors and outcome of treatment. Clin Infect Dis. 2009;49:1036–43. DOIPubMedGoogle Scholar
- Snowden KF. Microsporidia in higher vertebrates. In: Weiss LM, Becnel JJ, editors. Microsporidia: pathogens of opportunity, 1st edition. Chichester (UK): John Wiley & Sons, Inc.; 2014. p. 469–91.
- Fayer R, Santin‐Duran M. Epidemiology of microsporidia in human Infections. In: Weiss LM, Becnel JJ, editors. Microsporidia: pathogens of opportunity. Chichester (UK): John Wiley & Sons, Inc.; 2014. p. 135–64.
- Vávra J, Lukeš J. Microsporidia and ‘the art of living together’. Adv Parasitol. 2013;82:253–319. DOIPubMedGoogle Scholar
- Didier ES, Khan IA. The immunology of microsporidiosis in mammals. In: Weiss LM, Becnel JJ, editors. Microsporidia: pathogens of opportunity. Chichester (UK): John Wiley & Sons, Inc.; 2014. p. 307–26.
- Sak B, Kotková M, Hlásková L, Kváč M. Limited effect of adaptive immune response to control encephalitozoonosis. Parasite Immunol. 2017;39:
e12496 . DOIPubMedGoogle Scholar - Sak B, Brdíčková K, Holubová N, Květoňová D, Hlásková L, Kváč M. Encephalitozoon cuniculi genotype III evinces a resistance to albendazole treatment in both immunodeficient and immunocompetent mice. Antimicrob Agents Chemother. 2020;64:e00058–20. DOIPubMedGoogle Scholar
- Kotková M, Sak B, Kváč M. Differences in the intensity of infection caused by Encephalitozoon cuniculi genotype II and III - Comparison using quantitative real-time PCR. Exp Parasitol. 2018;192:93–7. DOIPubMedGoogle Scholar
- Gannon J. A survey of Encephalitozoon cuniculi in laboratory animal colonies in the United Kingdom. Lab Anim. 1980;14:91–4. DOIPubMedGoogle Scholar
- Mertens RB, Didier ES, Fishbein MC, Bertucci DC, Rogers LB, Orenstein JM. Encephalitozoon cuniculi microsporidiosis: infection of the brain, heart, kidneys, trachea, adrenal glands, and urinary bladder in a patient with AIDS. Mod Pathol. 1997;10:68–77.PubMedGoogle Scholar
- Weber R, Bryan RT, Schwartz DA, Owen RL. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–61. DOIPubMedGoogle Scholar
- Sak B, Kváč M. Chronic infections in mammals due to microsporidia. Exp Suppl. 2022;114:319–71. DOIPubMedGoogle Scholar
- Ditrich O, Chrdle A, Sak B, Chmelík V, Kubále J, Dyková I, et al. Encephalitozoon cuniculi genotype I as a causative agent of brain abscess in an immunocompetent patient. J Clin Microbiol. 2011;49:2769–71. DOIPubMedGoogle Scholar
- Couzinet S, Cejas E, Schittny J, Deplazes P, Weber R, Zimmerli S. Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes. Infect Immun. 2000;68:6939–45. DOIPubMedGoogle Scholar
- Nassonova ES, Tokarev YS, Trammer T, Entzeroth R, Sokolova YY. Phagocytosis of Nosema grylli (Microsporida, Nosematidae) spores in vivo and in vitro. J Eukaryot Microbiol. 2001;48(Suppl):83S–4S. DOIPubMedGoogle Scholar
- Sak B, Brady D, Pelikánová M, Květoňová D, Rost M, Kostka M, et al. Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J Clin Microbiol. 2011;49:1064–70. DOIPubMedGoogle Scholar
- Sak B, Kváč M, Kučerová Z, Květoňová D, Saková K. Latent microsporidial infection in immunocompetent individuals - a longitudinal study. PLoS Negl Trop Dis. 2011;5:
e1162 . DOIPubMedGoogle Scholar - Kotková M, Sak B, Květoňová D, Kváč M. Latent microsporidiosis caused by Encephalitozoon cuniculi in immunocompetent hosts: a murine model demonstrating the ineffectiveness of the immune system and treatment with albendazole. PLoS One. 2013;8:
e60941 . DOIPubMedGoogle Scholar - Konttinen YT, Pajarinen J, Takakubo Y, Gallo J, Nich C, Takagi M, et al. Macrophage polarization and activation in response to implant debris: influence by “particle disease” and “ion disease”. J Long Term Eff Med Implants. 2014;24:267–81. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: January 30, 2024
Table of Contents – Volume 30, Number 3—March 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Bohumil Sak, Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, Branišovská 31, České Budějovice 37005, Czech Republic
Top