Volume 30, Number 8—August 2024
Dispatch
Group B Streptococcus Sequence Type 103 as Human and Bovine Pathogen, Brazil
Abstract
Group B Streptococcus sequence type 103 is known primarily as a bovine mastitis pathogen. In Brazil, it has circulated in cattle and humans since the 1990s. It lacks scpB and, in humans, was found only among carriage isolates. Bovine–human interspecies transmission may have contributed to its evolution and spread.
Group B Streptococcus (GBS) is a major cause of life-threatening neonatal infections and bovine mastitis (1). The GBS population is composed of host-specialist lineages, such as sequence type (ST) 17, and host-generalist lineages, such as ST23 (2). ST103 is common among bovine GBS (bGBS) in Europe (3,4), Colombia (5), and China (6); reports of human GBS (hGBS) ST103 are rare and mostly limited to Asia (7–9). This difference may reflect limitations to host or geographic range or may be attributable to surveillance efforts. We investigated the molecular epidemiology of GBS STs in humans and cattle in Brazil.
We extracted genomic DNA from hGBS (carriage n = 416, disease n = 39) and bGBS (milk n = 151, environment n = 5) isolates collected in Brazil during 1978–2021 (Table 1) by using the DNeasy Blood & Tissue Kit (QIAGEN, https://www.qiagen.com). We generated whole-genome sequences at the Wellcome Sanger Institute (Hixton, UK; https://www.gbsgen.net) or at MicrobesNG (Birmingham, UK; https://microbesng.com) by using the Illumina NovaSeq platform (https://www.illumina.com). We used the sequences to predict ST, capsular types, antimicrobial resistance (AMR), and surface protein profiles (Alpha, Alp1, Alp2/3, Rib, Srr1, Srr2, pilus islands PI1, PI-2A1, PI-2A2, and PI-2B, and HvgA) with GBS Typer version 1.0.11 (https://github.com/sanger-bentley-group/GBS-Typer-sanger-nf), and to detect scpB gene carriage by using BLASTn (https://blast.ncbi.nlm.nih.gov) and GenBank reference sequence AF327852.1. We performed whole-genome alignment by using Snippy v4.6.0 (https://github.com/tseemann/snippy) and constructed a phylogenetic tree with RAxML as implemented in Gubbins version 3.3.1 (https://github.com/nickjcroucher/gubbins).
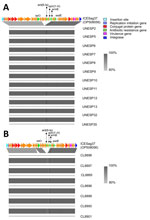
We included only GBS isolates assigned to ST103 in this study. Of the 611 isolates tested, 67 (11%) isolates belonged to ST103 (17 hGBS isolates, 1990–2020; 50 bGBS, 1999–2021) (Table 2), which showed that ST103 hGBS circulated in Brazil in parallel with bGBS well before it was reported elsewhere (7–9). In accordance with previous studies (2,4), ST103 isolates mostly belonged to serotype Ia, with 1 exception (hGBS, serotype II), which was also the only ssr1-negative isolate (Figure 1). Although rare, capsular switching can occur in GBS and may impair the effectiveness of future polysaccharide vaccines (10).
The core-genome phylogeny showed 10 clusters, largely representing either host or, for bGBS, farm of origin, and 3 singletons (Figure 1). Some bGBS (clusters D and E) were more closely related to hGBS (clusters B, C, and F) than to other bGBS (clusters G, I, and J) (Figure 1). The hGBS were distributed across 4 clusters and 3 singletons. Clusters B (n = 2 isolates), C (n = 2), and F (n = 8) formed a clade with bGBS clusters D and E and were detected throughout the period studied (1990–2020) (Figure 1). Cluster H (n = 2), which is located on a long branch in the phylogeny, included 2 historical strains isolated in 1990 from human oropharynx samples.
Some accessory genome traits were consistent across the entire phylogeny, whereas others differed between or within host species. All isolates carried pilus island PI-2B, as previously decribed (2,4). All hGBS and bGBS isolates lacked the scpB gene, which encodes C5a peptidase and is crucial for GBS adhesion and invasion of human cells (11). The absence of scpB gene may explain why ST103 was only found among carriage isolates in humans, although a larger sample size or in vitro studies would be needed to provide statistical or mechanistic support for that hypothesis.
The Alpha protein gene was absent in clusters nearest to the tree root, regardless of host, year or, for bGBS, farm of isolation, suggesting that the element was acquired later by the diverged subpopulation (clusters A–F). By contrast, the lactose operon was consistently present in all bGBS but not in all hGBS, suggesting either multiple loss or acquisition events in those host-associated clusters. The ability to ferment lactose, as mediated by the Lac.2 operon, drives GBS adaptation to the bovine udder (2). Presence of Lac.2 and the phenotypic ability to ferment lactose are relatively rare in human isolates (4,12). In our study, all bGBS and almost half of hGBS (n = 8, 47%) were able to ferment lactose, suggesting bovine-to-human transmission with subsequent loss of Lac.2 in the absence of selective pressure for its maintenance in the human niche. This finding contrasts with an earlier study, which suggested a human origin of bovine ST103 (3). In-depth analysis of the evolution of this clade using a larger collection of isolates across a broad geographic range would be needed to determine the timing and direction of host jumps.
We observed the greatest variability in accessory genome content in AMR genes. In bGBS, tetO was the most common resistance gene, followed by tetM, but never in combination. In hGBS, AMR genes were rarely found (Figure 1); this finding is in contrast to many other human-associated GBS lineages, which have a high prevalence of tetracycline resistance genes (13). In addition to tetO, bGBS clusters D and E also carried ermB and aadE on a variant of ICESag37 (OP508056), an integrative conjugative element associated with AMR and virulence genes, with a median nucleotide similarity of 86% (Figure 2). Overall, prevalence of AMR genes was higher in bGBS than in hGBS (88% vs. 17.6% of isolates carrying >1 AMR gene). Sampling bias might have contributed to this finding; several herds were represented by multiple isolates, which formed monophyletic clusters with homogeneous AMR profiles, as expected based on contagious transmission of bGBS within dairy herds. Nevertheless, we detected AMR in bGBS in every single herd, and AMR prevalence in bGBS would still be higher than in hGBS if each herd was represented by a single isolate, indicating a true biologic effect. The high prevalence of AMR among bGBS may be driven by the overuse of antimicrobial drugs in dairy herds in emerging economies like that of Brazil (1), where macrolides, tetracycline, and aminoglycosides are among the most commonly used antimicrobial drugs for treatment and prevention of clinical mastitis (14).
Vaccination is desirable to prevent hGBS and bGBS disease without reliance on antimicrobial drugs, but no human or bovine vaccines are licensed yet. Promising candidates for a human maternal vaccine include both polysaccharide-protein conjugate and protein subunit strategies. Surveillance of capsular types and surface proteins of emerging GBS lineages, including in low- and middle-income countries, is crucial to inform vaccine design, which can impair vaccine effectiveness. In our study, all ST103 strains were associated with serotype Ia and PI-2B, Srr1, and Alpha surface proteins, which are among the most immunogenic vaccine targets (15).
GBS is a multihost pathogen, able to adapt to different niches. The phylogeny of ST103 and the presence of the lactose operon in hGBS suggest that interspecies transmission (bovine-to-human) might have contributed to the evolution of ST103 in Brazil. In addition, our results suggest that the presence of the pilus variant PI-2B and absence of the scpB gene are common markers of ST103 in Brazil, irrespective of host species. The lack of scpB may limit the virulence of GBS ST103 in humans, which in turn may have contributed to its underreporting, especially in countries or studies that focus on clinical isolates. Additional studies at population and mechanistic level would be needed to fully understand the origin, evolution, epidemiology, and virulence potential of this emerging clade, but our results show that ST103 is more common in hGBS than previously described and that it has been circulating in Brazil at least since the 1990s.
Dr. Oliveira is a postdoctoral researcher at the Microbiology Institute of the Federal University of Rio de Janeiro, Brazil. Her primary research interests include investigating the molecular epidemiology, antimicrobial resistance markers, and virulence traits of GBS isolated from humans and animals to improve the surveillance and diagnosis of infectious diseases and to inform control strategies and vaccine design.
Acknowledgment
This work was supported by the International Veterinary Vaccinology Network (IVVN) Fellowship Programme and the JUNO project (https://www.gbsgen.net) through Bill and Melinda Gates Foundation grant (INV-010426).
References
- Oliveira LMA, Simões LC, Costa NS, Zadoks RN, Pinto TCA. The landscape of antimicrobial resistance in the neonatal and multi-host pathogen group B Streptococcus: review from a One Health perspective. Front Microbiol. 2022;13:
943413 . DOIPubMedGoogle Scholar - Richards VP, Velsko IM, Alam MT, Zadoks RN, Manning SD, Pavinski Bitar PD, et al. Population gene introgression and high genome plasticity for the zoonotic pathogen Streptococcus agalactiae. Mol Biol Evol. 2019;36:2572–90. DOIPubMedGoogle Scholar
- Crestani C, Forde TL, Lycett SJ, Holmes MA, Fasth C, Persson-Waller K, et al. The fall and rise of group B Streptococcus in dairy cattle: reintroduction due to human-to-cattle host jumps? Microb Genom. 2021;7:
000648 . DOIPubMedGoogle Scholar - Lyhs U, Kulkas L, Katholm J, Waller KP, Saha K, Tomusk RJ, et al. Streptococcus agalactiae serotype IV in humans and cattle, northern Europe. Emerg Infect Dis. 2016;22:2097–103. DOIPubMedGoogle Scholar
- Cobo-Ángel C, Jaramillo-Jaramillo AS, Lasso-Rojas LM, Aguilar-Marin SB, Sanchez J, Rodriguez-Lecompte JC, et al. Streptococcus agalactiae is not always an obligate intramammary pathogen: Molecular epidemiology of GBS from milk, feces and environment in Colombian dairy herds. PLoS One. 2018;13:
e0208990 . DOIPubMedGoogle Scholar - Yang Y, Liu Y, Ding Y, Yi L, Ma Z, Fan H, et al. Molecular characterization of Streptococcus agalactiae isolated from bovine mastitis in Eastern China. PLoS One. 2013;8:
e67755 . DOIPubMedGoogle Scholar - Boonyayatra S, Wongsathein D, Tharavichitkul P. Genetic relatedness among Streptococcus agalactiae isolated from cattle, fish, and humans. Foodborne Pathog Dis. 2020;17:137–43. DOIPubMedGoogle Scholar
- Shen L, Huang M, Xie N. Experimental study on Streptococcus agalactiae genotype and erythromycin resistance in neonatal sepsis. Cell Mol Biol. 2022;67:100–6. DOIPubMedGoogle Scholar
- Hsu JF, Chen CL, Lee CC, Lien R, Chu SM, Fu RH, et al. Characterization of group B Streptococcus colonization in full-term and Late-Preterm neonates in Taiwan. Pediatr Neonatol. 2019;60:311–7. DOIPubMedGoogle Scholar
- Bellais S, Six A, Fouet A, Longo M, Dmytruk N, Glaser P, et al. Capsular switching in group B Streptococcus CC17 hypervirulent clone: a future challenge for polysaccharide vaccine development. J Infect Dis. 2012;206:1745–52. DOIPubMedGoogle Scholar
- Franken C, Haase G, Brandt C, Weber-Heynemann J, Martin S, Lämmler C, et al. Horizontal gene transfer and host specificity of beta-haemolytic streptococci: the role of a putative composite transposon containing scpB and lmb. Mol Microbiol. 2001;41:925–35. DOIPubMedGoogle Scholar
- Sørensen UB, Poulsen K, Ghezzo C, Margarit I, Kilian M. Emergence and global dissemination of host-specific Streptococcus agalactiae clones. MBio. 2010;1:e00178–10. DOIPubMedGoogle Scholar
- Da Cunha V, Davies MR, Douarre PE, Rosinski-Chupin I, Margarit I, Spinali S, et al.; DEVANI Consortium. Streptococcus agalactiae clones infecting humans were selected and fixed through the extensive use of tetracycline. Nat Commun. 2014;5:4544. DOIPubMedGoogle Scholar
- Tomazi T, Dos Santos MV. Antimicrobial use for treatment of clinical mastitis in dairy herds from Brazil and its association with herd-level descriptors. Prev Vet Med. 2020;176:
104937 . DOIPubMedGoogle Scholar - McGee L, Chochua S, Li Z, Mathis S, Rivers J, Metcalf B, et al. Multistate, population-based distributions of candidate vaccine targets, clonal complexes, and resistance features of invasive group B streptococci within the United States, 2015–2017. Clin Infect Dis. 2021;72:1004–13. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: July 16, 2024
Table of Contents – Volume 30, Number 8—August 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Laura M.A. Oliveira, Av. Carlos Chagas Filho, 373 Bl I Room I02-028, Cidade Universitária, Instituto de Microbiologia Paulo de Goes, Universidade Federal do Rio de Janeiro, Rio de Janeiro 21941-902, Brazil
Top