Volume 30, Number 8—August 2024
Research Letter
Fecal Microbiota Transplantation for Severe Infant Botulism, China
Cite This Article
Citation for Media
Abstract
Infant botulism in a 4-month-old boy in China who continued to excrete toxins for over a month despite antitoxin therapy was further treated with fecal microbiota transplantation. After treatment, we noted increased gut microbial diversity and altered fecal metabolites, which may help reduce intestinal pH and enhance anti-inflammatory capabilities.
Infant botulism is caused by ingesting Clostridium botulinum spores and characterized by symmetric descending paralysis, which can progress to respiratory failure in severe cases (1). Specific therapies include intravenous administration of botulism immune globulin (BIG-IV or BabyBIG, https://infantbotulism.org/general/babybig.php) or botulinum antitoxin. However, BIG-IV and BabyBIG are unavailable in some countries, including China (2). Even after clinical signs have been alleviated through antitoxin therapy, some children may continue to excrete C. botulinum and its neurotoxin (BoNT) in their feces over a prolonged period, heightening the potential for relapse and transmission to others (although relatively rare) (3,4). Hence, effective treatments that promote clearance of intestinal C. botulinum spores are needed. During March–May 2021, we treated severe infant botulism in a 4-month-old boy in China who had continued excreting toxins for >1 month after clinical signs disappeared after antitoxin therapy. The study was approved by the Ethics Committee of Beijing Children’s Hospital (2023-E-149-R).
Five days before admission to Beijing Children’s Hospital (Beijing, China), the previously healthy infant exhibited intermittent fever, lethargy, poor appetite, and constipation, followed by respiratory distress. After intubation and mechanical ventilation in the emergency department, the patient was admitted to the pediatric intensive care unit. During examination, his pupils were dilated (≈4 mm) and had sluggish light reflexes but no signs of meningeal irritation. In addition, his muscle strength and tone were low. A series of tests excluded central nervous system infections, metabolic disorders, and other potential causes. By day 3 of hospitalization, the diagnosis of infant botulism was confirmed by detection of BoNT nucleic acid (serotype B) in fecal samples. Subsequently, we were able to obtain botulism antitoxin (monovalent type B) and administer it by intravenous injection of 2 mL (5,000 IU) 2×/day. Substantial improvement in clinical signs was observed by day 7 of hospitalization, and complete resolution was achieved by day 15.
Nevertheless, through day 33, multiple fecal samples tested for botulinum nucleic acid by real-time PCR and for BoNT by mouse bioassays (5) remained positive (Table). Consequently, during days 34–37 of hospitalization, the infant received a fecal microbiota transplantation (FMT; donor identification D024) at a dose of 20 mL via rectal enema daily for 4 consecutive days. During that time, he experienced low-grade fever and mild coughing, but his body temperature returned to reference range after FMT. After the third FMT (on day 36 after admission), test results for BoNT nucleic acid and mouse bioassays in feces were all negative (Table).
We conducted epidemiologic investigations for the exclusively breastfed patient and isolated C. botulinum from his home environment; serologic and genetic types matched those in his feces. After discharge, he was followed up for 6 months and exhibited good growth and development without any relapse.
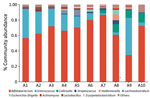
We analyzed gut microbiota composition and fecal metabolomics of 10 fecal samples (5 time points before and after FMT) (Figure; Appendix Figure 1). Use of 16S rRNA sequencing revealed increased α diversity of the gut microbiota after FMT, indicated by the Abundance-based Coverage Estimator and Shannon indices (6). At the genus level, relative abundance of Enterococcus and Lactobacillus was decreased and that of Collinsella and Holdemanella was increased. Although 16S rRNA sequencing cannot directly detect C. botulinum, the taxonomic group to which C. botulinum belongs (Clostridium_sensus_stricto_18) was also reduced. Ultra-high performance liquid chromatography–tandem mass spectrometry further revealed statistically significant alterations in 53 fecal metabolites after FMT; primarily implicated metabolic pathways were α-linolenic acid and linoleic acid metabolism, bile acid biosynthesis, bile acid metabolism, branched-chain amino acid metabolism, and butyrate metabolism. Correlation analyses indicated that changes in those metabolites were closely associated with alterations in the gut microbiota (Appendix Figure 2).
Infants with infant botulism may continue to excrete C. botulinum and BoNT for weeks or even months (7). The simpler gut microbiota with fewer taxa and the deficiency of bile acids in infants might contribute to the overgrowth of C. botulinum spores and their persistent excretion in the intestine (8). Our study illustrated increased gut microbiota diversity after FMT, concurrently accompanied by increased bile acid, potentially shifting the function of the microbiota–bile acid axis (9). Moreover, the increased production of metabolites, such as organic acids and docosapentaenoic acid, may help reduce intestinal pH and enhance anti-inflammatory capabilities (10). However, whether the restoration of gut microbiota diversity and changes in fecal metabolites are directly associated with the disappearance of C. botulinum spores is not clear. Because our study lacked a control group and solely represents an observational phenomenon, we cannot provide causal evidence for the effectiveness of FMT in treating infant botulism. Interventions such as fecal transplantation are recommended for children with infant botulism who continue to produce toxins for a long time.
Dr. Fan is currently an associate researcher at the Children's Hospital Affiliated with Capital Medical University, specializing in critical illness and infections in children.
Acknowledgments
We thank all nurses and doctors in the Beijing Children’s Hospital pediatric intensive care unit.
The study was funded by the National Key Research and Development Program of China (2021YFC2600501), Beijing Research Ward Project (BCRW202101), and Beijing Municipal Natural Science Foundation (7222057).
References
- Goldberg B, Danino D, Levinsky Y, Levy I, Straussberg R, Dabaja-Younis H, et al. Infant Botulism, Israel, 2007-2021. Emerg Infect Dis. 2023;29:235–41. DOIPubMedGoogle Scholar
- O’Horo JC, Harper EP, El Rafei A, Ali R, DeSimone DC, Sakusic A, et al. Efficacy of antitoxin therapy in treating patients with foodborne botulism: a systematic review and meta-analysis of cases, 1923-2016. Clin Infect Dis. 2017;66(suppl_1):S43–56. DOIPubMedGoogle Scholar
- Glauser TA, Maguire HC, Sladky JT. Relapse of infant botulism. Ann Neurol. 1990;28:187–9. DOIPubMedGoogle Scholar
- Aminian KSG, Gulati P. Recurrent infant botulism complicated by necrotizing enterocolitis. Pediatr Neurol. 2023;143:77–8. DOIPubMedGoogle Scholar
- Pellett S, Tepp WH, Johnson EA. Critical analysis of neuronal cell and the mouse bioassay for detection of botulinum neurotoxins. Toxins (Basel). 2019;11:713. DOIPubMedGoogle Scholar
- Wan Y, Yuan J, Li J, Li H, Yin K, Wang F, et al. Overweight and underweight status are linked to specific gut microbiota and intestinal tricarboxylic acid cycle intermediates. Clin Nutr. 2020;39:3189–98. DOIPubMedGoogle Scholar
- Yu PA, Lin NH, Mahon BE, Sobel J, Yu Y, Mody RK, et al. Safety and improved clinical outcomes in patients treated with new equine-derived heptavalent botulinum antitoxin. Clin Infect Dis. 2017;66(suppl_1):S57–64. DOIPubMedGoogle Scholar
- Huhtanen CM. Bile acid inhibition of Clostridium botulinum. Appl Environ Microbiol. 1979;38:216–8. DOIPubMedGoogle Scholar
- Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. DOIPubMedGoogle Scholar
- Liu Y, Wu H, Wang T, Shi X, He H, Huang H, et al. Paeonol reduces microbial metabolite α-hydroxyisobutyric acid to alleviate the ROS/TXNIP/NLRP3 pathway-mediated endothelial inflammation in atherosclerosis mice. Chin J Nat Med. 2023;21:759–74. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: July 10, 2024
1These authors contributed equally to this article.
Table of Contents – Volume 30, Number 8—August 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Xuefang Xu, National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, National Institute for Communicable Diseases Control and Prevention, Chinese Center for Disease Control and Prevention, Changping, Beijing 102206, China
Top