Volume 30, Number 8—August 2024
Dispatch
Highly Pathogenic Avian Influenza Virus A(H5N1) Clade 2.3.4.4b Infection in Free-Ranging Polar Bear, Alaska, USA
Abstract
We report a natural infection with a Eurasian highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in a free-ranging juvenile polar bear (Ursus maritimus) found dead in North Slope Borough, Alaska, USA. Continued community and hunter-based participation in wildlife health surveillance is key to detecting emerging pathogens in the Arctic.
Since its emergence in Europe during October 2020, highly pathogenic avian influenza (HPAI) A(H5N1) clade 2.3.4.4b virus has frequently spilled over into diverse mammal hosts globally. In North America, natural H5N1 infections have occurred in several bear species, including American black bears (Ursus americanus), Asiatic black bears (U. thibetanus), grizzly bears (U. arctos horribilis), and Kodiak brown bears (U. a. middendorffi) (1). Infections with influenza A(H1N1) viruses have been reported in captive sloth bears (Melursus ursinus) and Asiatic black bears (2,3) and in giant pandas (Ailuropoda melanoleuca) (4). Detection of hemagglutination inhibition antibodies against H3 and H6 subtype influenza viruses also suggested previous natural exposure to influenza viruses of avian origin (4). Seroconversion after natural exposure to bird influenza viruses has been documented in the Barent Sea polar bear subpopulation (2010–2011) (5) and brown bears in Alaska (2013–2016) (6) but not in the southern Beaufort Sea polar bear subpopulation (2013–2016) (7). Polar bears are a threatened species under the US Endangered Species Act. We report and describe an infection by HPAI H5N1 virus in a free-ranging polar bear found dead in Alaska, USA, during 2023.
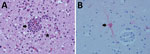
The North Slope Borough Department of Wildlife Management (NSB DWM) in Alaska conducts wildlife health research and maintains community-based harvest monitoring programs for marine mammals, including polar bears. The Alaska Office of the State Veterinarian conducts surveillance for notifiable infectious diseases in wildlife. After detecting HPAI H5N1 in birds of prey and a red fox (Vulpes vulpes) in April 2022, the Office of the State Veterinarian initiated collaborative surveillance testing with NSB DWM for avian influenza in birds and other wildlife. In August 2023, community members reported a dead polar bear without obvious external injuries near Point Barrow, Alaska (71°23′N, 156°28′W). At the NSB DWM laboratory in Utqiagvik, Alaska, we conducted a postmortem examination of the bear. The bear was young and male, 120 cm in body length, and in moderate to advanced decomposition. Body condition was fair to poor, with no back or visceral fat. Gross findings were multiple 1–3-cm ulcerative skin lesions around the left eye and oral commissure, liver and lung congestion, moderate sanguinal pericardial and cavitary effusion, cerebral swelling and congestion, and empty stomach. We collected postmortem tissue samples of the heart, lung, trachea, spleen, liver, kidney, adrenal gland, skin, skeletal muscle, mesenteric lymph node, pancreas, tongue, esophagus, stomach, small intestines, and brain (cerebrum) and fixed them in 10% neutral buffered formalin for 2 weeks. Histology Consultation Services (https://histocs.com) processed the tissue for routine histopathologic examination by staining with hematoxylin and eosin. We also collected oral, nasal, rectal, and brain swab samples and placed them in 2-mL cryovials, which we stored for 2 weeks at −50°C and shipped to the Alaska Environmental Health Laboratory in Anchorage, Alaska. Their personnel placed pooled swab specimens into brain–heart infusion broth. The primary histopathologic finding was a granulocytic and mononuclear meningoencephalitis with microgliosis, neuronal necrosis, neuronophagia, vasculitis, and parenchymal rarefaction (Figure, panel A). Other findings were pulmonary edema, focal lipid pneumonia, and multifocal ulcerative dermatitis.
Pooled swab specimens tested negative for the influenza virus matrix gene by PCR at the Washington Animal Disease Diagnostic Laboratory (Pullmans, WA, USA), which is a National Animal Health Laboratory Network facility. The root cause of negative PCR results is unclear because subsequent sequence analysis did not indicate assay failure. However, because of the cerebral lesions, we sent scrolls of formalin-fixed paraffin-embedded cerebral tissue to the Athens Disease Diagnostic Laboratory, University of Georgia (Athens, GA, USA), for immunohistochemistry to detect influenza A by using an influenza A virus polyclonal antibody (Abcam, https://www.abcam.com). Influenza A virus antigen was detected in cytoplasm of neurons and nuclei of microglial cells (Figure, panel B). We also sent scrolls of formalin-fixed paraffin-embedded cerebral tissue to the National Veterinary Services Laboratories (Ames, IA, USA), for molecular confirmation and virus genome characterization. HPAI virus genotype A3, a fully Eurasian influenza virus, was identified; this genotype was initially detected in Alaska in April 2022 and was the most frequently detected genotype in Alaska during August–December 2023. Reported markers for mammal adaptation were not identified. We deposited full genome sequences for the polar bear virus (A/polar bear/Alaska/23–0381234/2023) in GenBank (accession nos. PP820319–26) and GISAID (https://www.gisaid.org; accession no. EPI_ISL_18976667).
This detection of HPAI virus was in the southern Beaufort Sea subpopulation, 1 of 19 circumpolar polar bear subpopulations. During July–August 2023, three short-tailed shearwater seabirds (Ardenna tenuirostris) that tested positive for HPAI H5N1 clade 2.3.4.4b were found dead near Point Barrow, where the polar bear in this study was found. The shearwaters’ virus genotype shared 9 common single-nucleotide polymorphisms (SNPs) with the polar bear virus and was representative of the virus circulating in that area at the time rather than a direct source of the polar bear infection. In addition, in August 2023, a small mortality event from avian influenza occurred among common murre seabirds (Uria aalge) in Dillingham Census Area in Alaska; that virus genotype also shared 8–9 common SNPs, further supporting regional virus circulation. Polar bears are primarily dependent on seals as a food source but will prey on birds and eggs; thus, virus exposure from consumption of infected birds is possible, but infection via an olfactory route cannot be excluded (8).
Support does not exist for ongoing HPAI virus–associated illness and death in free-ranging polar bears in Alaska’s North Slope Borough; in 2023, swab specimens from 3 other dead polar bears tested negative for influenza virus by PCR (Appendix). As for black bears with HPAI H5N1 virus infections (9), brain lesions were the major histopathologic findings in this case. It is not unexpected for clade 2.3.4.4b virus–infected mammals with neurologic signs to have respiratory samples test negative (10), possibly because of different exposure routes, such as digestive, olfactory, or respiratory routes (8). The HPAI H5 goose/Guangdong lineage has been shown to be more neuropathogenic than other influenza A viruses in mammals (8), including the H5N1 clade 2.3.4.4b virus (11,12).
Other notable findings in this case were pulmonary edema, lipid pneumonia, and ulcerative skin lesions. Pulmonary edema as a gross lesion and on histologic analysis was a consistent finding in 3 domestic cats with H5N1 clade 2.3.4.4b infections (13) but has been infrequently reported in wild mesocarnivores (10). Lipid pneumonia was documented in subsistence-harvested polar bears in the southern Beaufort Sea and is considered unrelated (D. Rotstein, unpub. data). Ulcerative skin lesions not caused by trauma are rarely documented in subsistence-harvested southern Beaufort Sea polar bears (R. Stimmelmayr, unpub. data); those lesions have not been reported in terrestrial mammals infected with the H5 clade 2.3.4.4b lineage (10) but have been reported in pinnipeds infected with H3N8 virus (14).
Genome analysis of influenza viruses originating from wildlife in Alaska has shown both unreassorted and reassorted viruses. HPAI virus genotype A3 was likely introduced into Alaska via the East Asia–Australia Flyway as early as November 2021 (15) and has been detected in a few backyard premises; in many wild birds, including California condors (Gymnogyps californianus) in Arizona; and several mammals (red fox, fishers, martens, racoons, and brown bears) along the Pacific Flyway.
In the Arctic, wildlife and other wild subsistence foods play a pivotal role in the health, well-being, and food security of northern indigenous communities. Therefore, subsistence harvesting of animals infected with HPAI viruses, including polar bears, poses a zoonotic risk and affects traditional food safety and food security. Continued community and hunter-based participation in wildlife health surveillance is key to detecting emerging pathogens and other One Health issues in the Arctic.
Dr. Stimmelmayr is a wildlife veterinarian and research biologist with the North Slope Borough Department of Wildlife Management in Utqiagvik, Alaska. Her research interests focus on marine and terrestrial wildlife health and diseases within a One Health context of indigenous circumpolar hunting societies.
Acknowledgments
We thank the hunters and communities in the North Slope Borough who keep us informed about what they see when traveling the ocean and land; all of our subsistence and wildlife research assistant staff, as well as department leadership for their continued support of the NSB DWM wildlife health research program; Hon Ip for his support during the investigation; Histology Pathology Services for their slide preparation and rapid responsiveness; and staff in the Histology Laboratory in the Department of Pathology at the University of Georgia College of Veterinary Medicine for their immunohistochemistry expertise.
Marine mammal tissues were collected under US Fish and Wildlife Service (permit no. MA80164B-0).
The findings and conclusions in this publication are those of the authors and should not be construed to represent any official US Department of Agriculture or US Government determination or policy.
References
- US Department of Agriculture, Animal and Plant Health Inspection Service. Detections of highly pathogenic avian influenza in mammals [cited 2024 Feb 12]. https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/mammals
- Boedeker NC, Nelson MI, Killian ML, Torchetti MK, Barthel T, Murray S. Pandemic (H1N1) 2009 influenza A virus infection associated with respiratory signs in sloth bears (Melursus ursinus). Zoonoses Public Health. 2017;64:566–71. DOIPubMedGoogle Scholar
- Bessière P, Gaide N, Croville G, Crispo M, Fusade-Boyer M, Abou Monsef Y, et al. High pathogenicity avian influenza A (H5N1) clade 2.3.4.4b virus infection in a captive Tibetan black bear (Ursus thibetanus): investigations based on paraffin-embedded tissues, France, 2022. Microbiol Spectr. 2024;12:
e0373623 . DOIPubMedGoogle Scholar - Li D, Zhu L, Cui H, Ling S, Fan S, Yu Z, et al. Influenza A(H1N1)pdm09 virus infection in giant pandas, China. Emerg Infect Dis. 2014;20:480–3. DOIPubMedGoogle Scholar
- Naidenko SV, Ivanov EA, Mordvintsev IN, Platonov NG, Ershov RV, Rozhnov VV. Seropositivity for different pathogens in polar bears (Ursus maritimus) from Barents Sea Islands. Biol Bull Russ Acad Sci. 2013;40:779–82. DOIGoogle Scholar
- Ramey AM, Cleveland CA, Hilderbrand GV, Joly K, Gustine DD, Mangipane B, et al. Exposure of Alaska brown bears (Ursus arctos) to bacterial, viral, and parasitic agents varies spatiotemporally and may be influenced by age. J Wildl Dis. 2019;55:576–88. DOIPubMedGoogle Scholar
- Hemert CV, Spivey TJ, Uher-Koch BD, Atwood TC, Sinnett DR, Meixell BW, et al. Survey of Arctic Alaskan wildlife for influenza A antibodies: limited evidence for exposure of mammals. J Wildl Dis. 2019;55:387–98. DOIPubMedGoogle Scholar
- Bauer L, Benavides FFW, Veldhuis Kroeze EJB, de Wit E, van Riel D. The neuropathogenesis of highly pathogenic avian influenza H5Nx viruses in mammalian species including humans. Trends Neurosci. 2023;46:953–70.PubMedGoogle Scholar
- Jakobek BT, Berhane Y, Nadeau MS, Embury-Hyatt C, Lung O, Xu W, et al. Influenza A(H5N1) virus infections in 2 free-ranging black bears (Ursus americanus), Quebec, Canada. Emerg Infect Dis. 2023;29:2145–9. DOIPubMedGoogle Scholar
- Elsmo EJ, Wünschmann A, Beckmen KB, Broughton-Neiswanger LE, Buckles EL, Ellis J, et al. Highly pathogenic avian influenza A(H5N1) virus clade 2.3.4.4b infections in wild terrestrial mammals, United States, 2022. Emerg Infect Dis. 2023;29:2451–60. DOIPubMedGoogle Scholar
- Alkie TN, Cox S, Embury-Hyatt C, Stevens B, Pople N, Pybus MJ, et al. Characterization of neurotropic HPAI H5N1 viruses with novel genome constellations and mammalian adaptive mutations in free-living mesocarnivores in Canada. Emerg Microbes Infect. 2023;12:
2186608 . DOIPubMedGoogle Scholar - Bordes L, Vreman S, Heutink R, Roose M, Venema S, Pritz-Verschuren SBE, et al. Highly pathogenic avian influenza H5N1 virus infections in wild red foxes (Vulpes vulpes) show neurotropism and adaptive virus mutations. Microbiol Spectr. 2023;11:
e0286722 . DOIPubMedGoogle Scholar - Sillman SJ, Drozd M, Loy D, Harris SP. Naturally occurring highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b infection in three domestic cats in North America during 2023. J Comp Pathol. 2023;205:17–23. DOIPubMedGoogle Scholar
- Anthony SJ, St Leger JA, Pugliares K, Ip HS, Chan JM, Carpenter ZW, et al. Emergence of fatal avian influenza in New England harbor seals. MBio. 2012;3:e00166–12. DOIPubMedGoogle Scholar
- Youk S, Torchetti MK, Lantz K, Lenoch JB, Killian ML, Leyson C, et al. H5N1 highly pathogenic avian influenza clade 2.3.4.4b in wild and domestic birds: Introductions into the United States and reassortments, December 2021-April 2022. Virology. 2023;587:
109860 . DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: June 28, 2024
1These authors contributed equally to this article.
Table of Contents – Volume 30, Number 8—August 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Raphaela Stimmelmayr, North Slope Borough, Department of Wildlife Management, PO Box 69, Ahkovak St 1795, Utqiagvik, AK 99723, USA
Top