Detection of Nucleocapsid Antibodies Associated with Primary SARS-CoV-2 Infection in Unvaccinated and Vaccinated Blood Donors
Eduard Grebe
1
, Mars Stone
1, Bryan R. Spencer, Akintunde Akinseye, David J. Wright, Clara Di Germanio, Roberta Bruhn, Karla G. Zurita, Paul Contestable, Valerie Green, Marion C. Lanteri, Paula Saa, Brad J. Biggerstaff, Melissa M. Coughlin, Steve Kleinman, Brian Custer, Jefferson M. Jones, and Michael P. Busch
Author affiliations: Vitalant Research Institute, San Francisco, California, USA (E. Grebe, M. Stone, C. Di Germanio, R. Bruhn, K.G. Zurita, B. Custer, M.P. Busch); University of California, San Francisco (M. Stone, R. Bruhn, M.C. Lanteri, B. Custer, M.P. Busch); American Red Cross, Rockville, Maryland, USA (B.R. Spencer, P. Saa); Westat, Rockville (A. Akinseye, D. Wright); QuidelOrtho, Rochester, New York, USA (P. Contestable); Creative Testing Solutions, Tempe, Arizona, USA (V. Green, M.C. Lanteri); Centers for Disease Control and Prevention, Fort Collins, Colorado, USA (B.J. Biggerstaff); Centers for Disease Control and Prevention, Atlanta, Georgia, USA (M.M. Coughlin, J.M. Jones); University of British Columbia, Vancouver, British Columbia, Canada (S. Kleinman)
Main Article
Figure 2
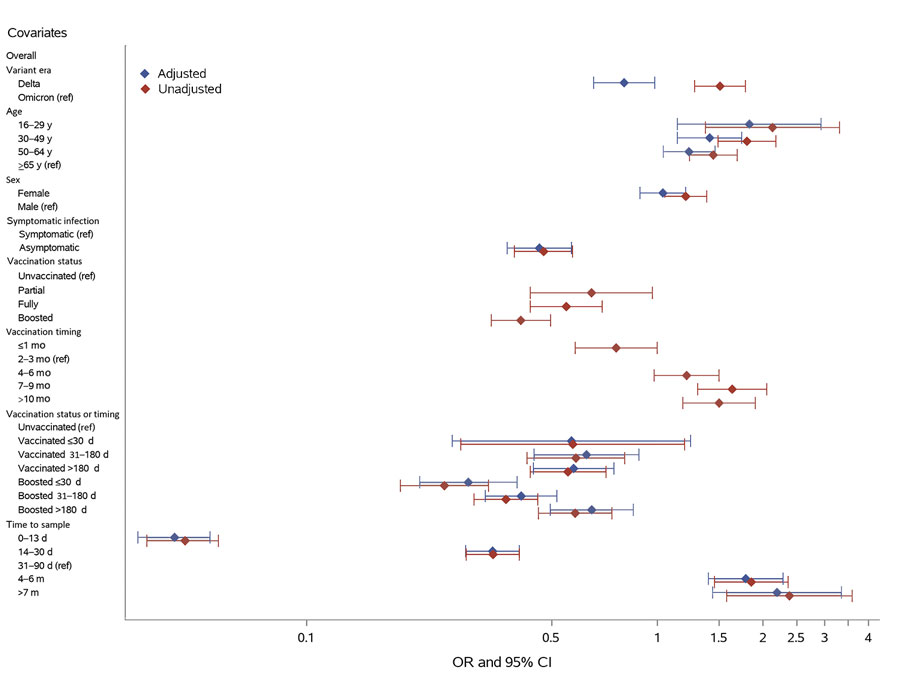
Figure 2. Factors influencing nucleocapsid antibody seroconversion after swab-confirmed first SARS-CoV-2 infections among vaccinated and unvaccinated blood donors, United States, July 2021–December 2022. ORs and 95% CIs from logistic regression are shown. In the multivariable regression model (adjusted ORs), the categories for certain variables have been grouped together; the vaccination status at the time of infection and timing of most recent vaccine before infection were combined in the vaccination status or timing variable, and in the variable for time from infection to tested sample, the groups for samples collected 7–12 months and >1 year postinfection were combined. Number of samples in each group, the proportion of nucleocapsid antibody–reactive samples, and ORs are shown in Appendix Table 2. OR, odds ratio; ref, referent.
Main Article
Page created: June 10, 2024
Page updated: June 11, 2025
Page reviewed: June 11, 2025
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
