Volume 30, Number 9—September 2024
Dispatch
Autochthonous Human Babesiosis Caused by Babesia venatorum, the Netherlands
Cite This Article
Citation for Media
Abstract
Severe babesiosis with 9.8% parasitemia was diagnosed in a patient in the Netherlands who had previously undergone splenectomy. We confirmed Babesia venatorum using PCR and sequencing. B. venatorum was also the most prevalent species in Ixodes ricinus ticks collected around the patient’s home. Our findings warrant awareness for severe babesiosis in similar patients.
Human babesiosis is a tickborne disease caused by protozoans that infect red blood cells. Immunocompromised persons and those who have undergone splenectomies are at risk for severe illness (1). Most confirmed cases of babesiosis in Europe have been in such patients and have most frequently been Babesia divergens infections (1). In the Netherlands, DNA of Babesia species have previously been reported in Ixodes ricinus ticks, blood of animals, and in several human samples (2–4). However, reports of autochthonous human babesiosis in the Netherlands are lacking. We report a case of autochthonous human babesiosis in the Netherlands, as well as data on Babesia DNA in ticks collected near the patient’s home. Handling of data and patient samples was performed according to the highest ethics standards. Informed consent was received from the patient.
In September 2023, a 69-year-old man was admitted to the hospital with fever (38°C), malaise, macroscopic hematuria, and laboratory results indicative of acute hemolysis. His medical history reported Hodgkin disease stage 3A in 1977, which went into remission after splenectomy and mantle field radiotherapy, and a diffuse large B-cell lymphoma in 2015, also in remission after being treated with 6 cycles of chemotherapy treatment. Several months before this hospitalization, chronic inflammatory demyelinating polyneuropathy had been diagnosed, and he was treated with immunoglobulins, plasma exchange, and dexamethasone pulse therapy.
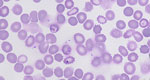
At the hospital, microscopy on peripheral blood smears revealed red blood cell inclusion bodies. The patient did not report recent travel to malaria-endemic areas but recalled a tick bite that occurred 2 months before near his home. Additional microscopy showed parasites with morphologic features that resembled Babesia spp.; 9.8% of red blood cells were infected (Figure 1). Immediately after admission, a red blood cell exchange transfusion and intravenous treatment with clindamycin and quinine reduced parasitemia to <1%. Treatment was switched to oral azithromycin and atovaquone. After 14 days, parasites were no longer detectable with microscopy. Babesia DNA remained detectable despite treatment until 4 months after admission. After proguanil was added to the treatment regimen, Babesia DNA disappeared the next week. Oral treatment was discontinued approximately 6 weeks later. No relapse of babesiosis has occurred, despite reinitiation of immunosuppressive drugs (Figure 2).
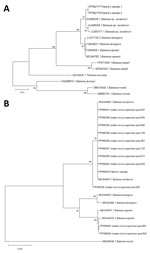
We visualized parasites using fluorescence microscopy on acridine-stained blood in quantitative buffy coat capillaries and conventional microscopy on Giemsa-stained thick and thin smears (Figure 1). Results of histidine-rich protein 2 and aldolase antigen testing (Biozek, https://www.biozek.com) and malaria loop-mediated isothermal amplification (Meridian Bioscience, https://www.meridianbioscience.com) were negative. A Plasmodium 18S ribosomal DNA PCR with known cross-reactivity with Babesia spp. was positive (5). Sanger sequencing of the ≈125 bp PCR amplicon revealed 100% homology with both B. divergens and B. venatorum. Babesia 18S ribosomal RNA (628 bp) amplification and sequencing showed 100% homology with B. venatorum (Figure 3, panel A) but not with B. divergens. Mitochondrial cytochrome oxidase subunit I (COI) amplification and sequencing showed 100% homology with B. venatorum from animals and ticks in the Netherlands (3). Species-specific PCRs for 4 Babesia spp. further confirmed the presence of B. venatorum DNA. A crude merozoite extract–based B. divergens ELISA showed optical densities slightly above the cutoff (6). Low titers might have resulted from continued plasmapheresis or poor cross-reactivity of antibodies against B. venatorum with B. divergens antigens.
To assess current and local risk of acquiring babesiosis, we collected questing ticks in 3 areas within 2–3.5 km of the patient’s home using blanket dragging (7). We selected the areas on the basis of the presence of (wild) ungulates, which are amplifying hosts of Babesia sensu stricto (3). Roe deer were present in all areas. In addition, areas 1 and 2 were grazed by fallow deer (Dama dama), domestic cattle (Bos taurus), and Konik horses (Equus caballus ferus). Of note, in area 1, a small herd of European bison (Bison bonasus) was introduced in 2007.
We collected a total of 2,786 I. ricinus ticks. Ticks were tested in pools because prevalence of Babesia DNA in ticks in the Netherlands is very low (3). If a pool was positive, only 1 tick was considered positive in that pool. Overall, 25 (0.9%) ticks were infected with Babesia s.s. based on amplification of mitochondrial COI (Table). From those Babesia-positive ticks, we amplified DNA with Babesia species–specific PCRs, which were positive for B. venatorum (n = 20), B. capreoli (n = 4), and the European variant of B. odocoilei (n = 1). Phylogenetic analysis demonstrated that the B. venatorum COI sequence from the patient clustered with B. venatorum sequences from ticks collected near the patient’s home (Figure 3, panel B). We calculated that 0.7% of ticks were infected with B. venatorum. The estimated incidence of tick bites in humans is 1.1 million/year, so human exposure to B. venatorum is ≈7,920 persons per year (8).
This case of autochthonous human babesiosis concerned life-threatening disease in a highly susceptible patient who had a rare medical history of diffuse large B-cell lymphoma years after Hodgkin disease. He was presumably infected with B. venatorum after a tick bite. The clinical course was favorable after prompt therapy, and parasites were undetectable after 2 weeks. Persistent Babesia DNA disappeared after adding proguanil to the treatment regimen. Of all Babesia DNA found in local ticks, B. venatorum was most prevalent, indicating a higher chance of exposure to this species. This finding is notable because, in Europe, most described human babesiosis has been caused by B. divergens, much less frequently by B. venatorum or B. microti (1).
In the Netherlands, DNA of >8 Babesia spp. have been found in animals and ticks but not in humans (2–4). Positive serologic results in studies among blood donors and persons with increased tick bite exposure indicate that self-limiting and unnoticed infections might occur in the general population in Europe (9,10). However, further investigations in such populations did not report Babesia DNA as evidence for human babesiosis (11,12). Scarcity of cases might also be explained by lack of awareness and underdiagnosis or misdiagnosis. Indeed, in several published cases, diagnosis was delayed or made postmortem (13). The morphologic overlap between Babesia and Plasmodium spp. in microscopy is high, making sensitive and specific testing for malaria imperative to prevent misdiagnosis and associated treatment delays. Last, underdiagnosis might also be the result of the high proportion of human babesiosis that could occur after unnoticed bites from nymphal ticks.
Different Babesia spp. are specific to different animal reservoirs. Therefore, determining infecting Babesia spp. can help assign surveillance among ticks and specific animals. A previous study conducted in the Netherlands during 2000–2019 found a widespread occurrence of B. venatorum DNA in 0.8% of 25,849 sampled I. ricinus ticks (3). B. venatorum was present in 46% (n = 290) of sampled roe deer (Capreolus capreolus) and to a much lesser extent in sheep (Ovis aries). Roe deer are highly present in the areas investigated for this case report, and the patient had observed many around his home. Tick densities could have increased in recent decades and changes in wildlife management could have contributed to spread of Babesia-infected ticks (14). Those changes might have driven wildlife infected with B. venatorum into areas where the parasite was formerly absent. Unfortunately, we were not able to sample local ungulates to investigate Babesia spp. prevalence. Finally, climate change might affect spatial and temporal tick distribution and increase the risk for and prevalence of human babesiosis, which warrants further surveillance studies (15). Although it remains unclear which factors have driven this occurrence of human babesiosis, awareness among clinicians is warranted, especially for susceptible patients.
Dr. Spoorenberg is an infectious diseases specialist at the Amsterdam University Medical Center, including in the Multidisciplinary Lyme Borreliosis Center. Her primary research interests are centered around clinical management of infectious diseases.
Acknowledgment
We thank Kimberly de Vries, Myrna Bouman, Leonie van Boetzelaer-Wittich, Ellen Wentink, Danielle Oude Velthuis, Carla Wassenaar, Chris van der Meer, and Leny Nieuwendijk for extensive microscopic analysis of all blood samples of the patient. The authors also thank Ankje de Vries, Manoj Fonville, Anna Rombouts, Amber van den Bron, Corine Nellestijn, Fion Brouwer, and Sophie van Tol for conducting fieldwork and Fokla Zorgdrager, Manoj Fonville, and Anne Wattimena for excellent detection and typing of Babesia spp. in the patient and in ticks. Finally, the authors thank Dieuwertje Hoornstra for delivering insights on unpublished prospective data on evidence of babesiosis in patients in the Netherlands and Gary Wormser for treatment advice.
References
- Bajer A, Beck A, Beck R, Behnke JM, Dwużnik-Szarek D, Eichenberger RM, et al. Babesiosis in southeastern, central and northeastern Europe: an emerging and re-emerging tick-borne disease of humans and animals. Microorganisms. 2022;10:945. DOIPubMedGoogle Scholar
- Wielinga PR, Fonville M, Sprong H, Gaasenbeek C, Borgsteede F, van der Giessen JW. Persistent detection of Babesia EU1 and Babesia microti in Ixodes ricinus in the Netherlands during a 5-year surveillance: 2003-2007. Vector Borne Zoonotic Dis. 2009;9:119–22. DOIPubMedGoogle Scholar
- Azagi T, Jaarsma RI, Docters van Leeuwen A, Fonville M, Maas M, Franssen FFJ, et al. Circulation of Babesia species and their exposure to humans through Ixodes ricinus. Pathogens. 2021;10:386. DOIPubMedGoogle Scholar
- Jahfari S, Hofhuis A, Fonville M, van der Giessen J, van Pelt W, Sprong H. Molecular detection of tick-borne pathogens in humans with tick bites and erythema migrans, in the Netherlands. PLoS Negl Trop Dis. 2016;10:
e0005042 . DOIPubMedGoogle Scholar - van Vugt M, Wetsteyn JC, Haverkort M, Kolader M, Verhaar N, Spanjaard L, et al. New England souvenirs. J Travel Med. 2011;18:425–6. DOIPubMedGoogle Scholar
- Tijani MK, Svensson J, Adlerborn P, Danielsson L, Teleka A, Lövmar ML, et al. How to detect antibodies against Babesia divergens in human blood samples. Open Forum Infect Dis. 2024;11:
ofae028 . DOIPubMedGoogle Scholar - Kohler CF, Sprong H, Fonville M, Esser H, De Boer WF, Van der Spek V, et al. Sand lizards (Lacerta agilis) decrease nymphal infection prevalence for tick-borne pathogens Borrelia burgdorferi sensu lato and Anaplasma phagocytophilum in a coastal dune ecosystem. J Appl Ecol. 2023;60:1115–26. DOIGoogle Scholar
- Hofhuis A, Harms M, van den Wijngaard C, Sprong H, van Pelt W. Continuing increase of tick bites and Lyme disease between 1994 and 2009. Ticks Tick Borne Dis. 2015;6:69–74. DOIPubMedGoogle Scholar
- Lempereur L, Shiels B, Heyman P, Moreau E, Saegerman C, Losson B, et al. A retrospective serological survey on human babesiosis in Belgium. Clin Microbiol Infect. 2015;21:96.e1–7. DOIPubMedGoogle Scholar
- Svensson J, Hunfeld KP, Persson KEM. High seroprevalence of Babesia antibodies among Borrelia burgdorferi-infected humans in Sweden. Ticks Tick Borne Dis. 2019;10:186–90. DOIPubMedGoogle Scholar
- Bloch EM, Siller A, Tonnetti L, Drews SJ, Spencer BR, Hedges D, et al. Molecular screening of blood donors for Babesia in Tyrol, Austria. Transfus Med Hemother. 2023;50:330–3. DOIPubMedGoogle Scholar
- Hoornstra D, Harms MG, Gauw SA, Wagemakers A, Azagi T, Kremer K, et al. Ticking on Pandora’s box: a prospective case-control study into ‘other’ tick-borne diseases. BMC Infect Dis. 2021;21:501. DOIPubMedGoogle Scholar
- Bläckberg J, Lazarevic VL, Hunfeld KP, Persson KEM. Low-virulent Babesia venatorum infection masquerading as hemophagocytic syndrome. Ann Hematol. 2018;97:731–3. DOIPubMedGoogle Scholar
- Sprong H, Hofhuis A, Gassner F, Takken W, Jacobs F, van Vliet AJ, et al. Circumstantial evidence for an increase in the total number and activity of Borrelia-infected Ixodes ricinus in the Netherlands. Parasit Vectors. 2012;5:294. DOIPubMedGoogle Scholar
- Drews SJ, Kjemtrup AM, Krause PJ, Lambert G, Leiby DA, Lewin A, et al. Transfusion-transmitted Babesia spp.: a changing landscape of epidemiology, regulation, and risk mitigation. J Clin Microbiol. 2023;61:
e0126822 . DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: August 14, 2024
1These authors contributed equally to this article.
Table of Contents – Volume 30, Number 9—September 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Rens Zonneveld, Department of Medical Microbiology and Infection Prevention, Amsterdam UMC, Meibergdreef 9, 1105 AZ, Amsterdam, the Netherlands
Top