Volume 31, Number 10—October 2025
Research Letter
Detection of Immunity Gap before Measles Outbreak, Ho Chi Minh City, Vietnam, 2024
Cite This Article
Citation for Media
Abstract
In 2022, we established a residual sample serosurveillance program in Ho Chi Minh City, Vietnam. During September 2022–April 2024, we found low measles antibody seroprevalence in children in the city’s western region, where a measles outbreak began in May 2024. Serosurveillance could be a useful tool for outbreak prediction and prevention.
Measles is a highly contagious vaccine-preventable disease. Ho Chi Minh City (HCMC), Vietnam, a city of 11 million persons, experienced a 20-month vaccination disruption during COVID-19 lockdowns and mass COVID-19 vaccination campaigns (June−November 2021) and postpandemic vaccine shortages (October 2022−December 2023). The last major measles outbreak in HCMC occurred in 2019. In 2024, measles cases increased in May, leading to an official outbreak declaration on August 27, 2024, in which 4,133 cases were reported by December 2024. Case-patients had a median age of 5.1 years (interquartile range 9 months to 12.6 years; range 1 month to 76 years). HCMC began a measles vaccination campaign on August 31, 2024, targeting children 1–5 years of age, and expanding to children 1–10 years of age on October 1, 2024.
Serosurveillance of antibody titers can be used to assess population immunity and relies on repeated serosurveys to monitor immunity changes over time (1). In HCMC, we established a serosurveillance system using anonymized residual serum samples from hospitals, cataloged and stored for future use. Sampling followed an age-stratified serial cross-sectional scheme every 3 to 4 months at Children’s Hospital 1, Children’s Hospital 2, and City Children’s Hospital. The only public pediatric hospitals in HCMC, those hospitals handle most hospitalized children 0–15 years of age across HCMC.
We analyzed 1,097 serum samples from children 0–15 years of age collected during September 2022−April 2024 by using a measles IgG ELISA assay (SERION Immunologics, https://www.serion-immunologics.com). We defined seropositivity as antibody titer >200 mIU/mL (sensitivity 99.0%; specificity 95.0%). We used generalized additive models with a binomial error distribution, logit link, and thin plate spline smooth terms to model seroprevalences as functions of age or time. For time analysis, the models applied population weights to each age. We corrected all models for sensitivity and specificity using the Rogan-Gladen estimate (2). We used line list data of patients residing in HCMC to investigate the outbreak. We estimated time-varying reproduction numbers, using previously described methods (3), with gamma-distributed serial intervals (mean 14.5 days; SD 3.25 days) (4) (Appendix).
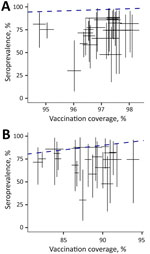
During September 2022−April 2024, seroprevalence of measles antibodies among children <15 years of age remained consistently <90% across all 3 hospitals (Figure 1). Such low seroprevalences in the 0–5-year age group, likely resulting from vaccination disruptions, translates to high risk for a measles outbreak. Seroprevalence was lower in the 5–15-year age group (Figure 1), possibly because of incomplete vaccination in the past. The western part of HCMC, corresponding to City Children’s Hospital catchment area (Figure 1), had the lowest seroprevalence and reported the first outbreak cases, highest cumulative incidence rates, and consistently high attack rates compared with other districts (Appendix Figure 1, panels A, C). Seroprevalence remained below the administrative coverage estimates for children who received both first and second measles vaccine doses (Figure 2; Appendix Figure 2, Tables 1, 2).
Reproduction numbers peaked at 1.99 (95% CI 1.65–2.36) on August 13, 2024, and declined to 0.97–1.50 after the vaccination campaign (Appendix Figure 1). The percentage of infected patients 1–10 years of age decreased after the vaccination campaign targeting that group, and the percentage of infected 10–15-year-olds increased, and attack rates rapidly rose. Children 10–15 years of age and adults were infected later than other groups, but their attack rates increased quicker than for other age groups.
Measles antibody seroprevalence gaps might have served as an early warning for this measles outbreak. The lowest seroprevalence, observed in the city’s western region, corresponded to the earliest cases and highest attack rates. Low seroprevalence in persons 5–15 years of age might explain the number of measles cases in that group. Because that age group is the most socially connected in the country’s population (5), low immunity can accelerate community spread. Of note, researchers studying a 2014 measles outbreak in northern Vietnam recommended serosurveillance to assess outbreak risks and improve responses (6). Strengthening serosurveillance to detect immunity gaps early could enhance preparedness for future outbreaks.
The first limitation of our study is that hospital-based samples might underestimate community immunity. However, small pockets of susceptibility pose high outbreak risks, especially in communities with high average vaccination coverages (7). Estimating coverage from registry data is challenging because of variables such as uncertain residency and unrecorded migration from areas with lower vaccination coverage. Our hospital-based serosurveillance system is a valuable example of sentinel surveillance, especially where hospital-acquired infections have triggered previous outbreaks (8). A key advantage is efficiency and sustainability in sample collection. Second, although measles antibody titers correlate with protection, limited data are available to establish a protective threshold (9). We used the manufacturer-recommended cutoff of >200 mIU/mL to classify seropositivity.
In summary, our results suggest that low-cost, hospital-based serosurveillance data could detect risk for measles outbreaks >6 months in advance. Acknowledging that this approach to surveillance should be further validated to assess the specificity of such a system (false-positive alarms), we are confident that such an approach could effectively identify populations at high outbreak risk, potentially informing optimal vaccination strategies.
Dr. Thinh Ong is a medical doctor and a DPhil student at the University of Oxford, based at the Oxford University Clinical Research Unit, Ho Chi Minh City, Vietnam. His research interests focus on using mathematical modelling and surveillance systems to improve forecasting and enhance strategies for infectious disease elimination.
Acknowledgments
We thank Children’s Hospital 1, Children’s Hospital 2, and City Children’s Hospital for providing the residual serum samples.
This study was approved by the institutional review board of the Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam (approval no. 1735/QD-BVBND), and the Oxford Tropical Research Ethics Committee (OxTREC reference no. 577-24), University of Oxford, United Kingdom.
L.V.T. is supported by Wellcome (grant no. 226120/Z/22/Z). The OUCRU serum bank, measles assays, T.O., P.N.T.N., H.N.N., and M.C. are supported by Wellcome through the core grant of OUCRU (no. 225167/A/22/Z).
References
- Metcalf CJE, Farrar J, Cutts FT, Basta NE, Graham AL, Lessler J, et al. Use of serological surveys to generate key insights into the changing global landscape of infectious disease. Lancet. 2016;388:728–30. DOIPubMedGoogle Scholar
- Diggle PJ. Estimating prevalence using an imperfect test. Epidemiol Res Int. 2011;2011:
608719 . DOIGoogle Scholar - Cori A, Ferguson NM, Fraser C, Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178:1505–12. DOIPubMedGoogle Scholar
- Worden L, Ackley SF, Zipprich J, Harriman K, Enanoria WTA, Wannier R, et al. Measles transmission during a large outbreak in California. Epidemics. 2020;30:
100375 . DOIPubMedGoogle Scholar - Prem K, Zandvoort KV, Klepac P, Eggo RM, Davies NG, Cook AR, et al.; Centre for the Mathematical Modelling of Infectious Diseases COVID-19 Working Group. Projecting contact matrices in 177 geographical regions: An update and comparison with empirical data for the COVID-19 era. PLoS Comput Biol. 2021;17:
e1009098 . DOIPubMedGoogle Scholar - Choisy M, Trinh ST, Nguyen TND, Nguyen TH, Mai QL, Pham QT, et al. Sero-prevalence surveillance to predict vaccine-preventable disease outbreaks; a lesson from the 2014 measles epidemic in Northern Vietnam. Open Forum Infect Dis. 2019;6:ofz030. DOIGoogle Scholar
- Truelove SA, Graham M, Moss WJ, Metcalf CJE, Ferrari MJ, Lessler J. Characterizing the impact of spatial clustering of susceptibility for measles elimination. Vaccine. 2019;37:732–41. DOIPubMedGoogle Scholar
- Tran DM, Ong T, Cao TV, Pham QT, Do H, Phan PH, et al. Hospital-acquired infections and unvaccinated children due to chronic diseases: an investigation of the 2017-2019 measles outbreak in the northern region of Vietnam. BMC Infect Dis. 2024;24:948. DOIPubMedGoogle Scholar
- Bolotin S, Hughes SL, Gul N, Khan S, Rota PA, Severini A, et al. What is the evidence to support a correlate of protection for measles? A systematic review. J Infect Dis. 2020;221:1576–83. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: September 18, 2025
Table of Contents – Volume 31, Number 10—October 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Thinh Ong, Oxford University Clinical Research Unit, 764 Vo Van Kiet St, Ward 1, District 5, Ho Chi Minh City 70000, Vietnam
Top