Volume 31, Number 10—October 2025
Dispatch
Fatal Pneumocephalus Caused by Hypervirulent Klebsiella pneumoniae, Germany
Abstract
We report a fatal case of pneumocephalus in Germany caused by hypervirulent Klebsiella pneumoniae sequence type 23, confirmed by using clinical, histopathologic, and genomic analyses. The patient reported no travel history, suggesting local emergence. This unusual case reveals an unclear pathogen prevalence and demonstrates the need for increased awareness of global spread.
The global spread of hypervirulent Klebsiella pneumoniae from Asia poses a major risk to human health that requires constant surveillance (1). Those strains are characterized by severe clinical manifestations in previously healthy patients or the presence of convergence plasmids conferring virulence and multidrug resistance that causes difficult-to-treat infections. In this article, we describe a fatal hypervirulent K. pneumoniae infection in Germany.
A 71-year-old patient was hospitalized with signs of septic shock. Thoracic and abdominal computed tomography (CT) imaging revealed pneumonia and liver and prostate abscesses. We initiated intravenous treatment with piperacillin/tazobactam and clarithromycin. Cranial CT revealed extensive pneumocephalus. We conducted CT by using the SOMATOM Force multidetector (Siemens, https://www.siemens.com) with secondary multiplanar reconstructions and maximum intensity projections. We conducted brain angiography natively and in the arterial phase after contrast medium application, and we conducted thoracic and abdominal imaging in the venous phase.
Three days after hospitalization, the patient died of septic shock with central hypoxia. We isolated hypermucoviscous K. pneumoniae from the patient’s blood cultures and postmortem brain samples. The fulminant clinical course justified extensive molecular biologic comparative analyses because similar cases were described previously in Asia (2).
We conducted an autopsy after the patient’s death. For postmortem histopathological workup at the Institute of Pathology at Ulm University Hospital (Ulm, Germany), we fixed the patient’s brain in 4% buffered formaldehyde and dissected according to our laboratory protocol. We paraffin-embedded tissue samples from 9 different regions according to our standard laboratory procedures. We conducted conventional (hematoxylin and eosin, Elastica van Gieson, Gram, periodic acid–Schiff, Berliner-Blau [Prussian blue]) and immunohistochemical (CD45, CD68, and GFAP proteins) staining on 3 -µm sections before microscopic evaluation.
We isolated K. pneumoniae from blood cultures by using the BACTEC system (BD, https://www.bd.com), postmortem swabs on casein-soy-peptone agar supplemented with 5% sheep blood (Oxoid, http://www.oxoidshop.com), and culture in brain–heart infusion broth (BD) for molecular workup. We used the Bruker (Bruker, https://www.bruker.com) matrix-assisted laser desorption/ionization time-of-flight mass spectrometry system for microbial identification and conducted antimicrobial testing by using VITEK2 (bioMérieux, https://www.biomerieux.com). We then conducted molecular analysis of 2 string test–positive isolates as previously described (3). For whole-genome sequencing, we extracted and quantified DNA by using the DNeasy Blood and Tissue Kit (QIAGEN, https://www.qiagen.com) and the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific, https://www.thermofisher.com). We prepared sequencing libraries by using a Nextera XT DNA Library Prep kit (Illumina, https://www.illumina.com) and conducted sequencing on an Illumina NextSeq 550 (Illumina) by using a v2.5 chemistry kit (2 × 150 bp). We performed genome trimming and assembly as previously described (3). We compared the 2 isolates from this patient (0354/24, 0355/25), an additional hypermucoviscous isolate from a patient with liver abscess in the same hospital (0074/24), and 26 recently published K. pneumoniae sequence type (ST) 23 isolates from hospitals in Germany (3,4) by using core genome multilocus sequence typing in SeqSphere 10.0.4 (Ridom, https://www.ridom.de) (5). We visualized the resulting tree with iTOL 6.8 (6). We identified contigs belonging to possible plasmids by MOBsuite 3.1.4 (5,7) and predicted resistance and virulence genes and capsular type by using Kleborate v3 (8,9).
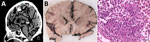
Clinical and radiologic findings supported the diagnosis of sepsis with central nervous system involvement. Although thoracic CT showed bronchial secretions and pulmonary consolidations, cranial CT revealed generalized hypoxic brain damage with intracranial gas inclusions (Figure 1, panel A). Abdominal CT revealed liver and prostate abscesses. On postmortem macroscopic examination, the brain showed signs of global hypoxic-ischemic brain damage, leptomeningitis, diffuse softening, oedematous swelling with signs of herniation (Figure 1, panel B), and numerous parenchymal gas inclusions. Histopathologic findings confirmed global hypoxic-ischemic damage and purulent leptomeningitis (Figure 1, panel C). Gram-stained sections from different brain areas contained gram-negative bacilli consistent with the microbiologic detection of hypermucoviscous K. pneumoniae from postmortem brain swab specimens. Hypoxic brain damage because of pneumocephalus caused by hematogenous dissemination of gas-producing bacteria was confirmed as the cause of death. No anatomic or functional anomalies were identified to explain the pneumocephalus. In addition, the autopsy confirmed a chronic liver abscess with incipient connective tissue encapsulation and partly abscessing, phlegmonous prostatitis with proof of K. pneumoniae.
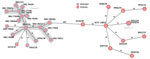
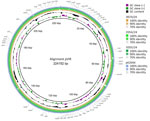
Genome analyses of the 2 hypermucoviscous K. pneumoniae isolates from blood cultures detected the magA gene, which is specific for capsule type K1, and a characteristic combination of virulence-associated genes (iucA, iroB, peg-344, rmpA, and rmpA2) (10). Multilocus sequence typing assigned both isolates to ST23. Subsequent comparison with 27 other K. pneumoniae ST23 isolates from Germany with capsule types K1 and K57 revealed that the isolates from this case were genetically identical (minimum spanning tree cluster 2) but not closely related to the other isolates (Figure 2). Plasmid analysis identified a type AA406 virulence plasmid in the 2 isolates from this study and 25 of the 27 other ST23 isolates (Appendix Table). An alignment of the plasmids with the characteristic Pl virulence plasmid pK2044, which is almost exclusively found in ST23 isolates, confirmed a high sequence similarity (Figure 3) (10,11). In addition, 4 previous ST23-K57 isolates carried a convergence plasmid (AA405) containing both virulence-associated and antimicrobial resistance genes, highlighting the emerging clinical significance of ST23 (3,4).
We describe a lethal pneumocephalus case caused by hypervirulent K. pneumoniae ST23 in Germany. Similar cases were reported from Southeast Asia (13). To date, we have found only sporadic reports of hypervirulent and mostly imported K. pneumoniae strains in Europe (3). Of note, our patient had no history of travel, and his only well-described risk factor was type 2 diabetes mellitus. Poor glycemic control increases the risk for fulminant K. pneumoniae infections with hematogenous dissemination; mortality rates of meningitis in such cases exceed 50% (1,14).
Despite early intensive care and antiinfective treatment, this patient died within 72 hours of hospitalization. The pathogenesis of the fulminant pneumocephalus is likely the consequence of septic dissemination from his primary infection site to the central nervous system with subsequent gas production. The patient’s K. pneumoniae ST23 isolates contained a virulence plasmid of type AA406 with typical virulence-associated genes and demonstrated no close genetic link to earlier reported K. pneumoniae ST23 strains in Germany (Figures 2,3).
This case indicates a potentially underestimated prevalence of hypervirulent ST23 strains in Europe, which requires systematic surveillance to better assess the distribution and epidemiology of K. pneumoniae ST23 (15) (https://op.europa.eu/en/publication-detail/-/publication/1e194058-d79e-11ee-b9d9-01aa75ed71a1). Clinicians should consider hypervirulent strains in cases of severe or recurrent K. pneumoniae infections, even without prior geographic exposure, and rapidly initiate microbiological diagnostics. Because not all hypervirulent K. pneumoniae strains are hypermucoviscous, and not all hypermucoviscous strains are hypervirulent (3), further molecular analyses should be implemented when hypervirulent K. pneumoniae infection is suspected. If molecular analysis is not possible despite clinical suspicion of infection with a hypervirulent strain, clinical management should be adjusted quickly (e.g., identification of occult abscesses).
Because of the dynamic global spread of pathogens, clinical and laboratory diagnostics are critical to efficient surveillance strategies for emerging pathogens such as hypervirulent K. pneumoniae, the epidemiologic relevance of which might still be underestimated. Further research is needed to clarify the reasons for different clinical courses in infections with hypervirulent K. pneumoniae isolates despite harboring identical virulence factors.
Against the background of globalization, complex trading networks, and increasing travel activities, enhanced genomic surveillance is crucial to detect and respond to novel pathogen threats such as hypervirulent K. pneumoniae. Close interdisciplinary collaboration between clinicians, pathologists, epidemiologists, and microbiologists is essential for a comprehensive understanding and sound surveillance practices.
Mr. Gläser is a medical student and doctoral candidate at the Institute of Orthopedic Research and Biomechanics at Ulm University, Germany. His academic interests include clinical research and supporting other students in planning, running, and publishing their own clinical studies.
Acknowledgments
We thank Sibylle Müller-Bertling and Kirstin Ganske for their excellent technical assistance. We thank the members of the Sequencing Core Facility of the Genome Competence Centre, Robert Koch Institute, for providing excellent sequencing service.
Author contributions: conceptualization, N.G., N.S.; methodology, A.E., A.O., J.B., J.B.H., Y.P.; validation, A.W., M.A.F., Y.P.; formal analysis, A.W., M.A.F.; investigation, A.E., A.O., A.W., M.A.F., Y.P.; resources, A.E., Y.P.; data curation, A.W., M.A.F.; writing: original draft preparation, A.W., J.B.H., N.G., N.S., Y.P.; writing: review and editing, A.E., A.I., A.O., J.B., M.A.F., T.F.E.B.; visualization, A.W., Y.P.; supervision, J.B.H.; project administration, A.I., J.B.H., Y.P. All authors have read and provided written consent to publish the current version of the manuscript.
References
- Das M. Global update on hypervirulent Klebsiella pneumoniae. Lancet Infect Dis. 2024;24:
e621 . DOIPubMedGoogle Scholar - Lee EJ, Kim RO, Lee M, Joo BE. Concurrent spontaneous pneumocephalus and subarachnoid hemorrhage due to Klebsiella pneumoniae meningitis. J Clin Neurol. 2022;18:253–5. DOIPubMedGoogle Scholar
- Wahl A, Fischer MA, Klaper K, Müller A, Borgmann S, Friesen J, et al. Presence of hypervirulence-associated determinants in Klebsiella pneumoniae from hospitalised patients in Germany. Int J Med Microbiol. 2024;314:
151601 . DOIPubMedGoogle Scholar - Sandfort M, Hans JB, Fischer MA, Reichert F, Cremanns M, Eisfeld J, et al. Increase in NDM-1 and NDM-1/OXA-48-producing Klebsiella pneumoniae in Germany associated with the war in Ukraine, 2022. Euro Surveill. 2022;27:
2200926 . DOIPubMedGoogle Scholar - Robertson J, Nash JHE. MOB-suite: software tools for clustering, reconstruction and typing of plasmids from draft assemblies. Microb Genom. 2018;4:
e000206 . DOIPubMedGoogle Scholar - Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–6. DOIPubMedGoogle Scholar
- Robertson J, Bessonov K, Schonfeld J, Nash JHE. Universal whole-sequence-based plasmid typing and its utility to prediction of host range and epidemiological surveillance. Microb Genom. 2020;6:mgen000435. DOIGoogle Scholar
- Lam MMC, Wick RR, Watts SC, Cerdeira LT, Wyres KL, Holt KE. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun. 2021;12:4188. DOIPubMedGoogle Scholar
- Wyres KL, Wick RR, Gorrie C, Jenney A, Follador R, Thomson NR, et al. Identification of Klebsiella capsule synthesis loci from whole genome data. Microb Genom. 2016;2:
e000102 . DOIPubMedGoogle Scholar - Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. DOIPubMedGoogle Scholar
- Wu KM, Li LH, Yan JJ, Tsao N, Liao TL, Tsai HC, et al. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J Bacteriol. 2009;191:4492–501. DOIPubMedGoogle Scholar
- Russo TA, Lebreton F, McGann PT. A step forward in hypervirulent Klebsiella pneumoniae diagnostics. Emerg Infect Dis. 2025;31:1–3. DOIPubMedGoogle Scholar
- Marr CM, Russo TA. Hypervirulent Klebsiella pneumoniae: a new public health threat. Expert Rev Anti Infect Ther. 2019;17:71–3. DOIPubMedGoogle Scholar
- Chang WN, Huang CR, Lu CH, Chien CC. Adult Klebsiella pneumoniae meningitis in Taiwan: an overview. Acta Neurol Taiwan. 2012;21:87–96.PubMedGoogle Scholar
- Neumann B, Stürhof C, Rath A, Kieninger B, Eger E, Müller JU, et al. Detection and characterization of putative hypervirulent Klebsiella pneumoniae isolates in microbiological diagnostics. Sci Rep. 2023;13:19025. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: September 17, 2025
1These first authors contributed equally to this article.
Table of Contents – Volume 31, Number 10—October 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jürgen Benjamin Hagemann, Ulm University Hospital Institute of Medical Microbiology and Hygiene, Albert-Einstein-Allee 23 (M23/4202), D-89081 Ulm, Germany
Top