Volume 31, Number 10—October 2025
Research Letter
Neonatal Gonococcal Conjunctivitis Caused by Fluoroquinolone-Resistant Neisseria gonorrhoeae
Cite This Article
Citation for Media
Abstract
Prophylaxis for ophthalmia neonatorum remains in use despite decreased incidence of the condition. We report a breakthrough case of neonatal conjunctivitis in Japan caused by a levofloxacin-resistant Neisseria gonorrhoeae bacteria strain, co-infected with Chlamydia trachomatis bacteria. This case highlights failures in screening, prophylaxis, and treatment, underscoring the need to reassess prevention strategies.
Since the introduction of prophylactic ophthalmic solutions by Carl Credé in 1881, the incidence of neonatal gonococcal conjunctivitis has declined markedly (1,2). Over time, the agents used for ocular prophylaxis have shifted from silver nitrate to erythromycin or tetracycline ophthalmic ointments and povidone/iodine (3).
In Japan, erythromycin/colistin ophthalmic formulations had been widely used since 1970. However, production of erythromycin/colistin preparations was discontinued in 2015, prompting some institutions to adopt fluoroquinolone-based ophthalmic agents for neonatal prophylaxis. Although fluoroquinolone-resistant Neisseria gonorrhoeae bacteria strains have been reported in adults, neonatal infections with such strains remain rare (2). We describe a breakthrough case of neonatal gonococcal conjunctivitis caused by a fluoroquinolone-resistant N. gonorrhoeae strain and further complicated by concurrent Chlamydia trachomatis bacteria infection. This case highlights the need to reevaluate current strategies for preventing and managing neonatal conjunctivitis.
In 2023, a 12-day-old female infant was brought to a hospital in Shizuoka, Japan, where she was observed to have purulent ocular discharge and periorbital swelling. She was born at full-term through spontaneous vaginal delivery. The mother had a negative C. trachomatis nucleic acid amplification test at 12 weeks’ gestation; however, no screening for N. gonorrhoeae was performed. The infant received prophylactic levofloxacin ophthalmic solution immediately after birth. From day 4 of life, purulent ocular discharge started occurring and progressively worsened, despite use of levofloxacin ophthalmic solution, applied 3 times daily, that was prescribed by a local pediatrician on day 8. By day 10, bilateral eyelid edema and marked periorbital inflammation were evident, prompting referral to the hospital’s ophthalmology department.
We obtained conjunctival cultures and added a cephalosporin-based ophthalmic preparation to the patient’s treatment plan. Culture of the ocular discharge confirmed N. gonorrhoeae infection, leading to a diagnosis of neonatal gonococcal conjunctivitis. We then admitted the infant for treatment.
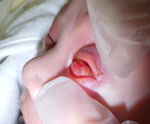
At admission, we recorded vital signs, laboratory test results, and urine test results (Appendix). Physical examination revealed bilateral conjunctival injection, pronounced left eyelid edema, and periorbital erythema (Figure). The corneas were clear, having no evidence of epithelial defect, ulceration, or perforation. We noted no anterior chamber abnormalities, and ocular motility was preserved. We observed a conjunctival hemorrhage in the left eye.
We initiated intravenous cefotaxime after hospitalization. We discontinued antimicrobial therapy after 48 hours of negative blood culture results. Ocular symptoms began improving by hospital day 3.
A PCR test performed on a pharyngeal swab sample on hospital day 3 was positive for C. trachomatis, and we administered azithromycin. The infant recovered fully without recurrence.
The N. gonorrhoeae isolate, which we designated as B196-JP22, was resistant to levofloxacin (MIC 12 μg/mL), had elevated MIC for azithromycin (MIC 0.75 μg/mL), and had reduced susceptibility to penicillin G (1 μg/mL) (Table). We performed full-genome analysis on the isolate (Appendix) and deposited it to the National Center for Biotechnology Information BioProject database (project no. PRJNA1277472) and BioSample database (accession no. SAMN49109531). We found missense mutations in the gyrA and parC genes consistent with the levofloxacin-resistance phenotype (5) and antimicrobial resistance genes (Table) (Appendix).
According to the multilocus sequence typing analysis at the Center of Genomic Epidemiology (https://www.genomicepidemiology.org) the sequence type was 7371 (Appendix Table 1, 2). The phylogenetic tree clustered our B196-JP22 isolate in a monophyletic terminal clade together with a urogenital strain (BioProject no. PRJNA560592; BioSample accession no. SAMN12591021) isolated in the city of Shenzhen, Guangdong Province, China, in January 2017 (6). Isolates in neighboring terminal clades were reported from Australia, Hong Kong, mainland China, and Vietnam (Appendix Figure).
This case demonstrates a triple failure: inadequate maternal screening for sexually transmitted infections and ineffective prophylaxis and treatment using fluoroquinolone-based ophthalmic agents against a multidrug-resistant N. gonorrhoeae strain. Robust maternal screening is critical to prevent perinatal transmission of sexually transmitted infections. US Centers for Disease Control and Prevention guidelines recommend repeat testing for N. gonorrhoeae and C. trachomatis during the second trimester for all pregnant women <25 years of age at increased risk (7). In contrast, prenatal care in Japan typically involves a single screening early in pregnancy, and routine screening for N. gonorrhoeae is uncommon (8).
In developed countries, the necessity of routine neonatal ocular prophylaxis is increasingly debated because it does not prevent C. trachomatis infection and the number of cases of gonococcal conjunctivitis is small (9). Several countries in Europe have discontinued prophylaxis without observing increased incidence of neonatal ophthalmia (10).
In Japan, fluoroquinolone-resistant N. gonorrhoeae strains are highly prevalent among adults; resistance rates are >80% for fluoroquinolones and ≈20% for macrolides (2). Genomic analysis of our isolate suggests further spread of resistant organisms in Asia, highlighting the importance of identifying this isolate in the region.
Given the low incidence of neonatal gonococcal conjunctivitis and the increasing prevalence of antimicrobial resistance in Japan, routine use of prophylactic ophthalmic solutions appears insufficient. Instead, systematic screening for maternal gonococcal infection, especially in late pregnancy, might be considered a more effective strategy to prevent vertical transmission.
Dr. Mizushima is a fellow in pediatric infectious diseases at Tokyo Metropolitan Children’s Hospital. His interests are the epidemiology and prevention of pediatric infectious diseases.
Acknowledgments
The study was funded by Japan’s Ministry of Health, Labour and Welfare (grant nos. MHLW 23HA1002 and 30 E-1, awarded to I.M.). Written permission for publication was obtained from the parents of the patient. An artificial intelligence tool (Chat GPT-4o, https://openai.com/index/hello-gpt-4o) was used only for grammatical correction of the manuscript.
All authors declare that they do not have any potential, perceived, or real conflicts of interest relevant to this study. H.M. and I.M. wrote the first draft of the manuscript, and C.A.Y performed the analysis of the isolate. M.K. provided data. All authors have critically reviewed the paper and are accountable for all aspects of the work, meeting International Committee of Medical Journal Editors criteria for authorship.
References
- Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al.; US Preventive Services Task Force. Ocular prophylaxis for gonococcal ophthalmia neonatorum: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2019;321:394–8. DOIPubMedGoogle Scholar
- Kazuaki K, Shogo O, Ken S, Hideyuki T, Masatomo M, Makoto O, et al. A case of neonatal gonococcal conjunctivitis suggesting the need for appropriate culture tests. Journal of the Japanese Society for Clinical Microbiology. 2021;31:22–6.
- Kapoor VS, Evans JR, Vedula SS. Interventions for preventing ophthalmia neonatorum. Cochrane Database Syst Rev. 2020;9:
CD001862 .PubMedGoogle Scholar - Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 35th edition. CLSI standard M100. Wayne (PA): The Institute; 2025.
- Belland RJ, Morrison SG, Ison C, Huang WM. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone-resistant isolates. Mol Microbiol. 1994;14:371–80. DOIPubMedGoogle Scholar
- Zhang C, Wang F, Zhu C, Xiu L, Li Y, Li L, et al. Determining antimicrobial resistance profiles and identifying novel mutations of Neisseria gonorrhoeae genomes obtained by multiplexed MinION sequencing. Sci China Life Sci. 2020;63:1063–70. DOIPubMedGoogle Scholar
- Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1–187. DOIPubMedGoogle Scholar
- Kawaguchi R, Matsumoto K, Ishikawa T, Ishitani K, Okagaki R, Ogawa M, et al. Guideline for gynecological practice in Japan: Japan Society of Obstetrics and Gynecology and Japan Association of Obstetricians and Gynecologists, 2020 edition. J Obstet Gynaecol Res. 2021;47:5–25. DOIPubMedGoogle Scholar
- Franco S, Hammerschlag MR. Neonatal ocular prophylaxis in the United States: is it still necessary? Expert Rev Anti Infect Ther. 2023;21:503–11. DOIPubMedGoogle Scholar
- Tzialla C, Auriti C, Aversa S, Merazzi D, Martinelli S, Araimo G, et al.; On Behalf Of Their Respective Scientific Societies. Intersociety position statement on the prevention of ophthalmia neonatorum in Italy. Microorganisms. 2023;12:15. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: September 18, 2025
Table of Contents – Volume 31, Number 10—October 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Isao Miyairi, Department of Pediatrics, Hamamatsu University School of Medicine, 1-20-1 Handayama, Chuo-ku, Hamamatsu, Shizuoka 435-3192, Japan
Top