Volume 31, Number 11—November 2025
Dispatch
Bjerkandera spp. Pulmonary Infection in Immunocompromised Hosts, Germany
Abstract
We report 3 cases of probable invasive pulmonary disease caused by Bjerkandera spp. fungi in immunocompromised patients in Germany. Accurate identification required internal transcribed spacer sequencing. Response to antifungal treatment varied. Our report underlines the pathogenic potential of Bjerkandera spp. and the importance of molecular diagnostics in rare fungal infections.
Common molds such as Aspergillus spp. and Mucorales are well-recognized pathogens in immunocompromised patients that cause life-threatening invasive fungal disease (IFD). Other environmental molds are frequently dismissed as contaminants in clinical specimens, yet growing evidence through clinical vigilance and advances in molecular techniques has revealed some as emerging threats in vulnerable populations (1–2). Many of those fungi are expected to remain unidentified because cultures stay negative without prolonged incubation for those organisms, and accurate identification requires molecular methods. Advanced molecular methods, such as cell-free DNA sequencing, hold promise as diagnostic tools but are not yet routinely available (3,4).
Bjerkandera spp., including B. adusta (syn. Geotrichopsis mycoparasitica) and B. fumosa, are filamentous basidiomycetes, wood-decaying fungi that have been isolated from dead hardwood trees in Europe and South America (5). Bjerkandera spp. have been linked to chronic cough, allergic bronchopulmonary mycosis, and hypersensitivity pneumonitis in humans (6–8). In addition, invasive sinonasal fungal disease by B. adusta was reported in a patient with uncontrolled type 2 diabetes, confirmed through histopathologic examination (9). We describe 3 patients in Germany with pulmonary infection and identification of Bjerkandera spp. in respiratory specimen that meet the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) criteria for probable IFD, highlighting an emerging association between the basidiomycete and human invasive disease.
Patient 1 was a 32-year-old man who received allogeneic hematopoietic stem cell transplant (HSCT) for relapsed mediastinal T-cell lymphoma (Table). Nine months later, he experienced progressive dyspnea. The patient was on prednisolone (100 mg/d) immunosuppressive therapy and did not receive antifungal drugs. Computed tomography (CT) of the chest showed new ground-glass opacitis and nodular consolidations. Culture from bronchoalveolar lavage (BAL) fluid revealed a mold identified as Bjerkandera spp. by sequencing the internal transcribed spacer (ITS) 1/2 region in accordance with Clinical and Laboratory Standards Institute guidelines (10). We did not perform susceptibility testing because fungal growth on the testing media was insufficient. Results of Mucorales and Aspergillus-specific PCR from BAL fluid were negative. Serum was negative for galactomannan antigen. We did not perform BAL galactomannan testing. We identified no other potential causes of infectious diseases by culture, PCR, or serology (Table). We initiated empiric antimicrobial therapy with piperacillin/tazobactam for 2 weeks. The fungus was not considered clinically significant; no antifungal treatment was initiated. Immunosuppression was intensified on suspicion of lung graft-versus-host disease, but the patient’s condition continued to deteriorate. We started antifungal therapy with posaconazole both as prophylaxis and targeted treatment of the probable IFD. Eight days later, we performed another BAL in which no fungus or other infectious agent was detected. The patient died shortly afterward from respiratory failure. No autopsy was performed.
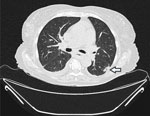
Patient 2 was an 82-year-old woman who was referred to the University Hospital Cologne with a diagnosis of acute myeloid leukemia 5 months before admission (Table). She had been treated with azacitidine monotherapy, but after allergic transfusion reaction to platelets, her cancer treatment was discontinued. At admission, the patient was experiencing hyperleukocytosis and neutropenia. We initiated cytoreductive treatment with hydroxyurea. A baseline chest CT scan revealed nodular infiltrates and a subpleural cavitary lesion, suggestive of fungal pneumonia (Figure 1). Of note, the patient had not received any antifungal prophylaxis other than trimethoprim/sulfamethoxazole. Bronchoscopy demonstrated purulent secretions. Results of galactomannan testing of BAL fluid were positive; culture yielded a preliminary phenotypic identification of Geotrichum spp. All other diagnostic work-up results were unremarkable (Table). Given the clinical significance of the mold identification, we pursued further species-level analysis. ITS sequencing identified the organism as either B. adusta or B. fumosa (10). Aspergillus PCR was negative. We could not perform antifungal susceptibility testing because of insufficient fungal growth. We initiated voriconazole therapy but switched to isavuconazole because the patient experienced visual disturbances. Follow-up chest CT scans at 2 and 5 weeks showed stable disease. After 5 weeks of antifungal therapy, we discontinued treatment and initiated posaconazole as secondary prophylaxis. No additional follow-up visits occurred.
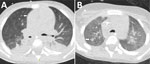
Patient 3 was a 4-year-old boy with newly diagnosed acute myeloid leukemia who initially received cytarabine-based induction chemotherapy (Table). He experienced seizures secondary to cerebral hemorrhage, requiring mechanical ventilation. After successful extubation, we resumed chemotherapy using a high-dose cytarabine and mitoxantrone regimen. He received micafungin (4 mg/kg 2×/wk) as antifungal prophylaxis. Subsequently, he experienced febrile neutropenia (temperature 39.2°C/102.6°F) that was unresponsive to empiric broad-spectrum antimicrobial treatment with meropenem and vancomycin, raising suspicion for IFD. We initiated voriconazole. Serum galactomannan test results were negative. A chest CT scan demonstrated nodular infiltrates and a new cavitary lesion radiographically consistent with a mold infection (Figure 2, panel A). We switched antifungal therapy to liposomal amphotericin B (L-AmB). Fungal culture from a tracheal aspirate yielded a mold with sterile mycelium, which we identified via ITS sequencing as B. adusta or B. fumosa (10). Other diagnostic assessments yielded no findings (Table). After 2 weeks of L-AmB therapy, the patient experienced severe hypokalemia, requiring a switch back to voriconazole. A follow-up CT scan performed 3 weeks later demonstrated a radiographic response to treatment (Figure 2, panel B). No further imaging was done. The patient continued voriconazole therapy for a total of 4 months.
We described 3 cases with probable invasive lung infections caused by Bjerkandera spp. in 2 adults and 1 pediatric patient with hematologic malignancies. We identified no other fungal pathogens or alternative infectious agents by culture, PCR, or serology. All cases met criteria for probable invasive pulmonary mold infection (11). Multidisciplinary teams discussed the possibility of contamination and likelihood of invasive disease by Bjerkandera spp. and concluded that the identification of Bjerkandera spp. was consistent with an IFD in each case, warranting antifungal treatment.
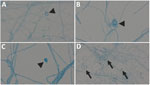
We identified the fungus in all 3 cases by sequencing techniques, underlining the importance of molecular approaches in the evaluation of rare fungal infections. In patient 2, B. adusta was preliminary identified as Geotrichum spp. based on phenotypic appearance. Geotrichum spp. are environmental fungi that cause opportunistic infections in at-risk populations (12). Both fungi share phenotypic features such as whitish, fluffy to woolly colony morphology and wide-branching septate hyphae with formation of arthroconidia and only occasional formation of chlamydospores (Figure 3) (6,13). Therefore, reliable identification in filamentous basidiomycetes requires additional techniques such as matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and sequencing methods. Sequence analysis of the ITS ribosomal DNA has better accuracy for species identification; however, for rare fungi, reference data may be incomplete or unavailable for both methods (14).
Guidance for clinical management of emerging IFD remains limited because IFD is rare and clinical manifestations vary. Susceptibility test results of 14 B. adusta isolates included high MIC for fluconazole and flucytosine and low MIC for AmB and newer triazoles (15). Clinical improvement with itraconazole treatment has been described (14) in cases with chronic cough associated with Bjerkandera spp., consistent with in vitro susceptibility findings. One reported case-patient with invasive rhinosinusitis caused by Bjerkandera was treated sequentially with L-AmB, posaconazole, and voriconazole, leading to clinical recovery (9). We used newer triazoles and L-AmB for treatment with variable responses. The lack of comprehensive susceptibility testing and outcome data limits definitive treatment recommendations for suspected IFD caused by Bjerkandera spp. Describing an unusual pathogen carries a risk for error. We were unable to demonstrate fungal growth in independent respiratory specimens or to obtain histologic proof of invasive growth from lung biopsy.
Our findings suggest that Bjerkandera spp. is a human pathogen causing invasive fungal pneumonia or other pulmonary infection in persons at risk, including the immunocompromised. Evaluating the clinical relevance of such infections must consider the degree of immunosuppression and the patient’s future treatment plans.
Dr. Sprute is a physician-scientist at University Hospital Cologne specializing in translational approaches to the management of invasive fungal and other opportunistic infections. Her clinical and research focus centers on high-risk patients, particularly those with hematologic malignancies and in intensive care units.
Acknowledgments
We thank our patients and their legal guardians for consenting to publication and providing detailed information on their courses of treatment. We thank Anna Dudakova, Tamara Rügamer, and Michaela Simon from the Institute of Medical Microbiology, Immunology, and Hygiene, University Hospital Cologne, for their valuable support in the phenotypic description of Bjerkandera spp. and for kindly providing microscopic images.
FungiScope (http://www.fungiscope.net) is an international web-based registry for rare and emerging invasive fungal infections (http://www.clinicaltrials.gov, NCT 01731353). the Institutional Review Board and Ethics Committee of the University Hospital Cologne, Germany approved the FungiScope submission (study ID 05–102). Patient 1 has been documented in the FungiScope registry. We have obtained written and signed consent to publish the case report and images from patient 2 and the legal guardian of patient 3.
This study was carried out as part of our routine work.
References
- Del Principe MI, Seidel D, Criscuolo M, Dargenio M, Rácil Z, Piedimonte M, et al.; FUNGISCOPE (Global Emerging Fungal Infection Registry) and the SEIFEM (Sorveglianza Epidemiologica Infezioni nelle Emopatie). Clinical features and prognostic factors of Magnusiomyces (Saprochaete) infections in haematology. A multicentre study of SEIFEM/Fungiscope. Mycoses. 2023;66:35–46. DOIPubMedGoogle Scholar
- Sprute R, Salmanton-García J, Sal E, Malaj X, Ráčil Z, Ruiz de Alegría Puig C, et al.; FungiScope® ECMM/ISHAM Working Group. Invasive infections with Purpureocillium lilacinum: clinical characteristics and outcome of 101 cases from FungiScope® and the literature. J Antimicrob Chemother. 2021;76:1593–603. DOIPubMedGoogle Scholar
- Sprute R, Cornely OA, Chen SC, Seidel D, Schuetz AN, Zhang SX. All you need to know and more about the diagnosis and management of rare yeast infections. mBio. 2021;12:
e0159421 . DOIPubMedGoogle Scholar - Sprute R, Seidel D, Cornely OA, Hoenigl M. EHA endorsement of the global guideline for the diagnosis and management of rare mold infection: an initiative of the European Confederation of Medical Mycology in cooperation with International Society for Human and Animal Mycology and American Society for Microbiology. Hemasphere. 2021;5:
e519 . DOIPubMedGoogle Scholar - Motato-Vásquez V, Gugliotta AM, Rajchenberg M, Catania M, Urcelay C, Robledo G. New insights on Bjerkandera (Phanerochaetaceae, Polyporales) in the Neotropics with description of Bjerkandera albocinerea based on morphological and molecular evidence. Plant Ecol Evol. 2020;153:229–45. DOIGoogle Scholar
- Chowdhary A, Kathuria S, Agarwal K, Meis JF. Recognizing filamentous basidiomycetes as agents of human disease: A review. Med Mycol. 2014;52:782–97. DOIPubMedGoogle Scholar
- Ogawa H, Tone K, Fujimura M, Makimura K. Central suppressant therapies in unexplained chronic cough patients whose sputum cultures yielded Bjerkandera adusta. Allergol Int. 2019;68:125–6. DOIPubMedGoogle Scholar
- Tone K, Ogawa H, Gochi M, Nagano Y, Furube A, Inaki S, et al. Allergic bronchopulmonary mycosis associated with a novel pathogen: Bjerkandera adusta. Allergol Int. 2022;71:542–4. DOIPubMedGoogle Scholar
- Kurata Y, Kimizuka Y, Yaguchi T, Ito K, Yamamoto T, Serizawa Y, et al. Bjerkandera adusta fungi as causative agent of invasive chronic rhinosinusitis. Emerg Infect Dis. 2025;31:355–8. DOIPubMedGoogle Scholar
- Clinical Laboratory Standards Institute. Interpretive criteria for identification of bacteria and fungi by targeted DNA sequencing. 2nd edition (MM18). Wayne (PA): The Institute; 2018.
- Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–76. DOIPubMedGoogle Scholar
- Chen SC, Perfect J, Colombo AL, Cornely OA, Groll AH, Seidel D, et al. Global guideline for the diagnosis and management of rare yeast infections: an initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect Dis. 2021;21:e375–86. DOIPubMedGoogle Scholar
- Zhu HY, Shang YJ, Wei XY, Groenewald M, Robert V, Zhang RP, et al. Taxonomic revision of Geotrichum and Magnusiomyces, with the descriptions of five new Geotrichum species from China. Mycology. 2024;15:400–23. DOIPubMedGoogle Scholar
- Tone K, Ogawa H, Alshahni MM, Kuwano K, Makimura K. Real-time PCR detection of the basidiomycetous fungus Bjerkandera adusta: a tool to identify itraconazole responder patients with unexplained chronic cough. Respiration. 2019;97:84–91. DOIPubMedGoogle Scholar
- González GM, Sutton DA, Thompson E, Tijerina R, Rinaldi MG. In vitro activities of approved and investigational antifungal agents against 44 clinical isolates of basidiomycetous fungi. Antimicrob Agents Chemother. 2001;45:633–5. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: November 24, 2025
Table of Contents – Volume 31, Number 11—November 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Rosanne Sprute, Institute of Translational Research, Herderstrasse 52, 50931 Cologne, Germany
Top