Volume 31, Number 11—November 2025
Dispatch
Novel Dolphin Tupavirus from Stranded Atlantic White-Sided Dolphin with Severe Encephalitis, Canada, 2024
Figure 1
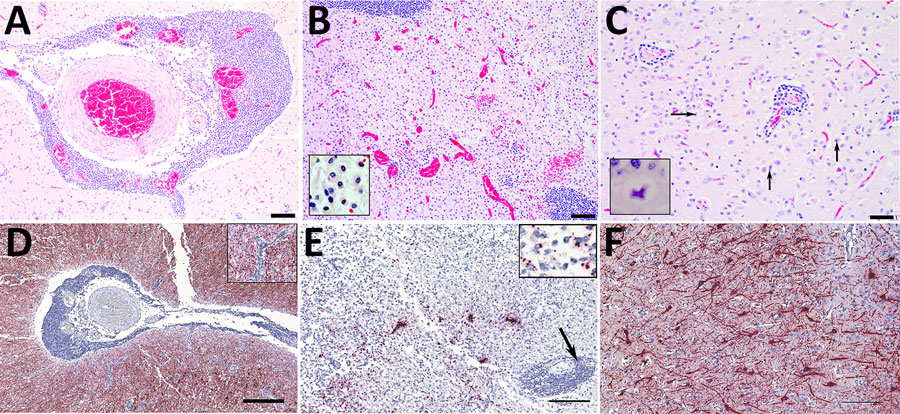
Figure 1. Histopathology (A–C) and in situ hybridization (D–F) of brain tissue from stranded Atlantic white-sided dolphin infected with novel tupavirus, Canada. Histopathology images are stained with hematoxylin and eosin; cytoplasm and connective tissues appear pink, red blood cells red, and cell nuclei purple. A) Cross-section through an arteriole within the neuropil at the level of the internal capsule, depicting sometimes massive infiltration of perivascular (Virchow-Robin) space by large numbers of lymphocytes and plasma cells, fewer numbers of macrophages, and rare eosinophils. Scale bar indicates 100 μm. B) Cross-section through the cingulate gyrus at the level of the internal capsule, showing almost complete effacement of normal neuropil architecture by massive numbers of hypertrophied microglial cells. Also depicted are multiple arterioles with large perivascular cuffs of lymphocytes and plasma cells, as well as multiple areas of hemorrhage within the neuropil. Inset shows microglial cells. Scale bar indicates 100 μm. C) Section through neocortex exhibiting extensive infiltration of the neuropil by glial cells, which frequently form nodules surrounding necrotic neurons. Necrotic neurons are shrunken with hypereosinophilic cytoplasm and condensed, pyknotic nuclei (arrows). There are also multiple small arterioles with lymphoplasmacytic perivascular cuffs. Inset shows a necrotic neuron. Scale bar indicates 50 μm. D) Abundant viral RNA (red staining) in the area surrounding the affected arteriole. Staining is observed in the neuropil as well as some glial cells; however, endothelial cells are not infected (inset). No staining is observed within the perivascular inflammatory cells. Scale bar indicates 400 μm. E) Viral RNA in microglial cells and neuropil (inset) but not within perivascular cuffs (arrow). Scale bar indicates 200 μm.) Abundant viral RNA within neurons and dendrites. In situ hybridization, paraffin-embedded formalin-fixed tissue sections were performed with RNAScope 2.5HD Detection Reagent and custom probes targeting the 1275–2283 nt region of the viral genome (Bio-Techne Advanced Cell Diagnostics, https://www.bio-techne.com). Scale bar indicates 200 μm.
1These authors contributed equally to this article.