Volume 31, Number 12—December 2025
Dispatch
Abnormal Prion Protein in Nasal Swab Specimens of Macaques Infected with Creutzfeldt-Jakob Disease
Cite This Article
Citation for Media
Abstract
We transfused 4 macaques with blood of macaques previously infected with variant Creutzfeldt-Jakob disease, transmitting disease transmittal to 2 macaques (1 demonstrating clinical signs). Nasal swab specimens from both infected macaques became positive for disease-associated prion protein during the preclinical stage. Such samples are suitable for antemortem diagnosis during long incubation periods.
Variant Creutzfeldt-Jakob disease (vCJD) and sporadic CJD are among a group of rare, always fatal neurodegenerative disorders known as transmissible spongiform encephalopathies (TSEs) or prion diseases (1). Researchers have linked vCJD to human dietary exposure to the agent that caused bovine spongiform encephalopathy in cattle (1–3). The literature documents few transfusion-transmitted cases of vCJD in the early 2000s (4,5), but no new cases have been reported since 2007. The lack of recent transfusion-transmitted vCJD is reassuring, but a question lingers about the true prevalence of vCJD infections in the population and the iatrogenic risk they pose. This prevalence is difficult to estimate without a validated assay to identify infected persons during the preclinical phase of vCJD, which can last for many decades.
To achieve this goal, we developed a nonhuman primate model of vCJD to collect specimens (blood and nasal swabs) suitable to validate preclinical tests and to detect the presence of abnormal disease-associated prion protein (PrPTSE) in blood (6,7). We could not conduct studies with human samples because of the rarity of human TSEs and the impossibility of establishing the exact time of most human exposures to the infective agents. To validate the relevance of a macaque model, we attempted to mimic human transmissions of transfusion-transmitted vCJD by blood transfusion from infected to naive macaques and collected biologic samples for testing over a 10-year period.
We transfused 4 uninfected macaques using blood from 3 macaques (CO7423, CO7422, and C16999) previously infected with vCJD (6) (Appendix). We collected 100-mL samples of blood from CO7423, at clinical onset and at terminal phase of illness, and immediately transfused the samples into 2 recipient macaques: CO1619 and 98CO19. Two years later, we transfused 2 macaques, DEIM and DFOO, with red blood cell–depleted blood prepared from whole blood of CO7422 and C16999 (Table 1). We euthanized CO1619 at 58 months posttransfusion (mpt) and euthanized 98CO19 at 104 mpt (8.7 years posttransfusion [ypt]) because of intercurrent illnesses (Table 1). DEIM developed early neurologic signs of mild ataxia and tremors at 104 mpt (8.7 ypt). Those symptoms slowly progressed to marked tremors, unsteadiness on the perch, unkept fur coat, and mild weight loss, all typical signs of vCJD in macaques. We euthanized DEIM 4 months after clinical onset. We euthanized DFOO at 120 mpt (10 ypt) when the macaque had reached the preselected experimental endpoint.
We collected blood and nasal swab specimens during and at the end of the study. We harvested brains and other tissues from each macaque. We also collected cerebrospinal fluid from DEIM and DFOO. To detect PrPTSE, the biomarker of TSEs, we used 2 in vitro assays: real-time quaking-induced conversion (RT-QuIC) to assay nasal swab extracts, lymph nodes, and cerebrospinal fluid; and protein misfolding cyclic amplification (PMCA) to assay brain, spleen, and blood samples (8,9). All tests of tissues and fluids from CO1619 and DFOO were negative for PrPTSE, including neuropathological examinations to show spongiform degeneration and PrPTSE deposits in formalin-fixed paraffin-embedded brain tissue (Table 2). We included samples from vCJD-infected animals and uninfected macaques in each test as controls.
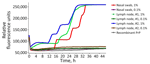
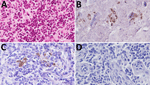
Brain homogenate from 98CO19 was negative for PrPTSE using multiple biochemical detection methods, and the macaque’s blood was negative by PMCA as well (Table 2). Neuropathologic studies showed no spongiform degeneration or PrPTSE deposits in 98CO19’s brain. However, nasal swab extracts and 2 inguinal lymph node homogenates collected at euthanasia were positive by RT-QuIC (Figure 1) when tested as 1% wt/vol tissue homogenates (4/4 positive wells) but negative in the next 10-fold dilution (0.1% wt/vol), suggesting that very small amounts of PrPTSE were present. PMCA assays of the same 2 tissues were negative. We noted scattered clusters of PrPTSE in sections of the lymph nodes, in the same cells staining for CD21, corresponding to follicular dendritic cells (Figure 2).
We confirmed macaque DEIM to be infected with vCJD by neuropathological and immunohistological examinations of formalin-fixed paraffin-embedded brain tissues and by Western blots of brain suspensions (Appendix Figure, panel A), RT-QuIC of cerebrospinal fluid, and PMCA of blood. PMCA first detected PrPTSE in blood collected at 85 mpt; RT-QuIC showed PrPTSE in nasal swab extracts 7 months later (92 mpt). PrPTSE signals remained positive in both tissues to the end of the study (Appendix Figure). Thus, PrPTSE appeared in those tissues several months before clinical onset, confirming the diagnostic potential of both assays with those materials.
We report the results of blood transfusions from 3 vCJD-infected macaques into 4 recipient macaques. One macaque developed clinical vCJD 9 years after transfusion, an interval consistent with a survival duration of 6.5–8.3 years seen in human recipients transfused with blood from asymptomatic vCJD-infected donors (5). One recipient macaque died early in the experiment, and 2 macaques survived ≥9 years with no clinical signs of vCJD. Macaque 98CO19 revealed PrPTSE signals in nasal swab extracts and inguinal lymph node tissues. Researchers have used lymph node tissue previously to identify macaques incubating vCJD before clinical onset of illness (11). Our data from RT-QuIC testing showed reactivity that was weak but unequivocal and reproducible. We confirmed the presence of PrPTSE in lymph node tissue by immunohistochemistry of the same tissue. We concluded that 98CO19 died while infected with vCJD.
Previous researchers exploring TTvCJD transfused to macaques reported a fatal neurologic syndrome, described as a myelopathy, that affected 3 of 7 macaques transfused with blood containing low infectivity (determined by low levels of peripheral PrPTSE); the other 4 macaques remained healthy (12). The macaques with myelopathy demonstrated clinical signs that included impaired visual acuity and hind limb ataxia, but tests revealed no PrPTSE in brain and other tissues (12). Macaque DFOO of our study showed no clinical signs associated with that described myelopathy. Macaque CO1619 died relatively early in the study—too early to know if it had been infected with vCJD or would have developed myelopathy. Our results largely agree with those of Comoy et al., who concluded that not all transfusions transmitted vCJD to macaques (12). Similarly, reports from the United Kingdom suggest that not all human recipients surviving 5 years or longer after transfusions developed TTvCJD from a donor with vCJD (13). No macaque in our smaller cohort of transfused animals showed evidence of myelopathy.
Over a 10-year period after transfusion, we collected relatively accessible biological materials, such as blood and nasal swab specimens, to test for PrPTSE. We first detected PrPTSE in the blood of macaque DEIM 19 months before onset of overt illness, consistent with results of our previous studies (6). Nasal swab extracts from the 2 infected macaques became positive for PrPTSE 12 months before signs of illness for DEIM and at euthanasia for 98CO19 (98CO19’s nasal swab specimens were negative 3 months earlier). Those results support potential use of nasal swab specimens as an assay matrix to identify infected persons before clinical onset of vCJD. A caveat is that we do not know how detection of PrPTSE might be affected by prion protein genotype. Research has demonstrated diagnostic accuracy of nasal swab testing for persons with either confirmed or presumed sporadic CJD, but the predictive value of this testing method for detecting cases before onset of neurologic illness remains uncertain (14). Acknowledging that our macaques were infected with vCJD, and not sporadic CJD, our results nonetheless suggest that testing nasal swab specimens to detect PrPTSE may be useful in screening persons with family history of CJD, which would enable attempts at early therapeutic intervention (15).
Dr. Juraj Cervenak is a biologist at the US Food and Drug Administration, Silver Spring, Maryland, USA. His research focuses on safety of biological products from contamination with transmissible spongiform encephalopathy agents to advance public health.
Acknowledgments
We thank the technical staff of the Nonhuman Primate Facility at Center for Biologics Evaluation and Research Division of Veterinary Services for their dedicated and excellent care of the animals. We are particularly indebted to John Dennis for his expertise in performing the macaque transfusions and Ruth Damaris Molano for providing expert veterinary care to the macaques. We also thank members of our laboratory for their support of this project.
The comments and opinions presented in this paper do not bind or obligate the US Food and Drug Administration.
References
- Will R. Variant Creutzfeldt-Jakob disease. Folia Neuropathol. 2004;42 Suppl A:77-83.
- Ironside JW. Variant Creutzfeldt-Jakob disease: an update. Folia Neuropathol. 2012;50:50–6.PubMedGoogle Scholar
- Belay ED, Schonberger LB. Variant Creutzfeldt-Jakob disease and bovine spongiform encephalopathy. [v–vi.]. Clin Lab Med. 2002;22:849–62, v–vi.PubMedGoogle Scholar
- Turner ML, Ludlam CA. An update on the assessment and management of the risk of transmission of variant Creutzfeldt-Jakob disease by blood and plasma products. Br J Haematol. 2009;144:14–23.PubMedGoogle Scholar
- Seed CR, Hewitt PE, Dodd RY, Houston F, Cervenakova L. Creutzfeldt-Jakob disease and blood transfusion safety. Vox Sang. 2018;113:220–31.PubMedGoogle Scholar
- Yakovleva O, Bett C, Pilant T, Asher DM, Gregori L. Abnormal prion protein, infectivity and neurofilament light-chain in blood of macaques with experimental variant Creutzfeldt-Jakob disease. J Gen Virol. 2022;103:
001764 .PubMedGoogle Scholar - McDowell KL, Nag N, Franco Z, Bu M, Piccardo P, Cervenak J, et al. Blood reference materials from macaques infected with variant Creutzfeldt-Jakob disease agent. Transfusion. 2015;55:405–12.PubMedGoogle Scholar
- Atarashi R, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods. 2007;4:645–50.PubMedGoogle Scholar
- Chen B, Morales R, Barria MA, Soto C. Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat Methods. 2010;7:519–20.PubMedGoogle Scholar
- Castilla J, Gutiérrez Adán A, Brun A, Pintado B, Ramírez MA, Parra B, et al. Early detection of PrPres in BSE-infected bovine PrP transgenic mice. Arch Virol. 2003;148:677–91.PubMedGoogle Scholar
- Lescoutra-Etchegaray N, Jaffré N, Sumian C, Durand V, Correia E, Mikol J, et al. Evaluation of the protection of primates transfused with variant Creutzfeldt-Jakob disease-infected blood products filtered with prion removal devices: a 5-year update. Transfusion. 2015;55:1231–41.PubMedGoogle Scholar
- Comoy EE, Mikol J, Jaffré N, Lebon V, Levavasseur E, Streichenberger N, et al. Experimental transfusion of variant CJD-infected blood reveals previously uncharacterised prion disorder in mice and macaque. Nat Commun. 2017;8:1268.PubMedGoogle Scholar
- Gregori L, Yang H, Anderson S. Estimation of variant Creutzfeldt-Jakob disease infectivity titers in human blood. Transfusion. 2011;51:2596–602.PubMedGoogle Scholar
- Fiorini M, Iselle G, Perra D, Bongianni M, Capaldi S, Sacchetto L, et al. High diagnostic accuracy of RT-QuIC assay in a prospective study of patients with suspected sCJD. Int J Mol Sci. 2020;21:880.PubMedGoogle Scholar
- Vallabh SM, Minikel EV, Schreiber SL, Lander ES. Towards a treatment for genetic prion disease: trials and biomarkers. Lancet Neurol. 2020;19:361–8.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: December 23, 2025
1Retired.
Table of Contents – Volume 31, Number 12—December 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Luisa Gregori, US Food and Drug Administration, 10903 New Hampshire Ave, Silver Spring, MD 20903, USA
Top