Volume 31, Number 12—December 2025
Dispatch
Bat Reovirus as Cause of Acute Respiratory Disease and Encephalitis in Humans, Bangladesh, 2022–2023
Cite This Article
Citation for Media
Abstract
We report 5 patients in Bangladesh presumed to have Nipah virus infections after consuming raw date palm sap. PCR and serology for Nipah virus were negative, but high-throughput sequencing identified Pteropine orthoreovirus in archived throat swab samples and virus cultures. This batborne virus should be considered in differential diagnosis of Nipah-like illnesses.
Bats are the natural reservoir of numerous known and novel zoonotic viruses, including rabies, Nipah, Hendra, Marburg, and severe acute respiratory syndrome viruses (1). In Bangladesh, Nipah virus (NiV) outbreaks are seasonal, and cases peak during December–April annually. In 2006, the Institute of Epidemiology, Disease Control and Research, Bangladesh (Dhaka, Bangladesh); icddr,b (Dhaka); and US Centers for Disease Control and Prevention (Atlanta, GA, USA) collaboratively established a hospital-based national sentinel surveillance program to address public health risks posed by NiV (2). During 2006–2022, the program enrolled >22,000 patients with symptoms of NiV infection. We report detection of Pteropine orthoreovirus (PRV) from 5 NiV-negative patients with acute respiratory disease and encephalitis during a 2022–2023 outbreak.
PRV is an emerging batborne orthoreovirus previously linked to acute respiratory infections in humans, especially in Southeast Asia (3–5). PRV is classified under the genus Orthoreovirus, family Reoviridae, which includes Nelson Bay virus (NBV), identified in Australia in 1968 (6). Zoonotic potential of NBV was confirmed in 2006, when a human case occurred in Melaka, Malaysia (7).
PRVs are nonenveloped, fusogenic viruses with double-stranded RNA genomes composed of 10 segments (S1, S2, S3, S4, M1, M2, M3, L1, L2, and L3). The S1 segment is tricistronic, encoding 3 proteins: cell-attachment protein, fusion-associated small transmembrane protein, and nonstructural protein p17 of unknown function (8).
The Bangladesh surveillance program uses quantitative PCR on throat swab samples to test for NiV RNA and on serum for NiV IgG or IgM. During December 2022–March 2023, five patients with presumptive NiV infection diagnoses were admitted to hospitals in Bangladesh but tested NiV-negative (Table). Three patients were admitted to Faridpur Medical College Hospital (MCH) (Faridpur, Bangladesh), and 1 patient each was admitted to Rajshahi MCH (Rajshahi, Bangladesh) and Khulna MCH (Khulna, Bangladesh). All patients had clinical signs and symptoms, including fever, disorientation, altered mental status, abnormal gait, and difficulty breathing. Four patients had a primary diagnosis of encephalitis, and 1 pediatric case had mild symptoms and a primary diagnosis of febrile convulsions (Table). All patients reported consuming raw date palm sap within 2 weeks of symptoms developing.
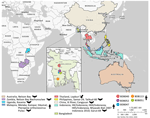
Patients originated from different geographic regions of Bangladesh. Case-patients BDB047, BDB051, and BDB052 were from Faridpur and Rajbari, within a 30-mile radius of central Bangladesh, near the Padma River Basin (Figure 1). Those 3 patients and pediatric case-patient BDB113 from Khulna (≈180 km south of Faridpur and Rajbari) were hospitalized within the same 2-week period in late December 2022 and early January 2023 (Table). Case-patient BDB040 was admitted in Sirajganj (≈150 km north of Faridpur and Rajbari) during March–April 2023. That patient had a history of chronic mental illness and also consumed raw date palm sap while hospitalized.
All patients were discharged after 2–3 weeks. During telehealth follow-up >15 months after discharge, case-patients BDB047 and BDB052 reported persistent fatigue, disorientation, and breathing and walking difficulties. Case-patients BDB051 and BDB113 fully recovered, but case-patient BDB040 died in August 2024, following deteriorating health and unexplained neurologic issues (Table).
We conducted viral discovery by using a capture-based agnostic viral sequencing method, VirCapSeq-VERT (VCS) (9), on total nucleic acid extracted from archived throat swab samples collected in viral-transport media. We perfomed VCS on NextSeq 2000 (Illumina, https://www.illumina.com), as previously described (9). We further used Megablast (MEGA, https://www.megasoftware.net) to compare retrieved sequences to those in GenBank nucleotide databases (Appendix). VCS analysis revealed PRV reads in all patients. We did not identify any other viral or bacterial pathogens in high-throughput sequencing.
We quantified viral load by using an in-house L2-based quantitative PCR (Appendix Table 1). Case-patient BDB051 had the highest viral load, likely due to the short (≈2-day) interval between raw date palm sap consumption and sample collection (Table). Case-patients BDB047 and BDB052 had higher viral loads than did BDB113 and BDB040.
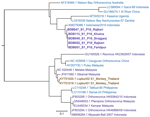
For phylogenetic analysis, we amplified the partial S1 segment encoding the p10 protein (96 aa) by using a consensus PCR (Figure 2; Appendix Table 2). The Bangladesh PRVs clustered at 99.3%–100.0% average nucleotide identity (ANI). Those PRVs showed ≈96% ANI with the Indonesia/2010 detected from a large flying fox (Pteropus vampyrus) in Indonesia, ≈85% ANI with the Nachunsulwe-57 detected from an Egyptian fruit bat (Rousettus aegyptiacus) in Zambia, and ≈77% ANI with the Kasama strain detected from an Angolan soft-furred fruit bat (Lissonycteris angolensis) in Uganda (10,11). The Bangladesh PRVs also had >77.0% ANI with Xi River virus from a fulvous fruit bat (R. leschenaultii) from China, Garut-69 virus from a large flying fox from Indonesia, and the 1968 prototypic NBV from a grey-headed flying fox (P. policephalus) in Australia.
To test whether molecular detection (VCS, quantitative PCR, and partial S1 amplicon sequencing) corresponded to infectious virus presence, we inoculated throat swab samples into MDCK cells and examined for cytopathic effects. After 2 MDCK passages, we passaged PRVs once in Vero cells. We successfully cultured virus from 3 swab samples (case-patients BDB047, BDB051, and BDB113) and sequenced on the MiSeq (Illumina) platform. We mapped reads to PRV genomes by using Geneious Prime (https://www.geneious.com) software.
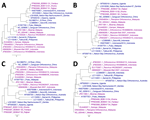
Complete coding sequences of all 10 Bangladesh PRV segments (GenBank accession nos. PP803379–408) showed 91.1%–100% ANI among themselves (Appendix Table 3). S1 segments showed 96.7%–99.9% ANI with each other and clustered with Indonesia/2010 strain, NBV-Australia, NBV-Nachunsulwe-57, and Kasama virus (Figure 3, panel A). Phylogeny of S2 and S3 segments were partially consistent with S1 segments (Figure 3, panels B, C). S4 segments clustered with Kampar and Melaka NBV strains (Figure 3, panel D), previously linked to mild respiratory illness in humans and reported human-to-human transmission (7,12).
L1, L2, L3, M1, M2, and M3 segments clustered with different PRVs isolated from fruit bats and occasionally from humans in Indonesia and Malaysia (Appendix Figure). That finding suggests unique evolution of each segment from reassortment events among strains circulating in Southeast Asia and long flight ranges of fruit bats. Reassortment is common for segmented RNA virus evolution and enhances risk for zoonotic potential (13). All Bangladesh PRV segments showed >76% ANI with NBV-Australia, exceeding the Internationl Committee for Taxonomy of Viruses 2022 species demarcation criteria of <75% ANI (14). Thus, the detected PRVs belong to NBV species but are distinct from other mammalian and avian reoviruses.
Humans in Bangladesh commonly consume raw date palm sap, especially in winter. Raw date palm sap is also a food source for fruit bats during winter and is the primary zoonotic route for NiV spillover from bats to humans (15). All 5 patients lived within 30–200 km of central Bangladesh but had no known contact with one another. Patients consumed raw date palm sap within 14 days of symptoms developing. Although no contemporaneous sap samples were available for analysis, we speculate that those PRV infections resulted from the consumption of raw date palm sap contaminated with bat excreta. All 5 patients had severe respiratory and neurologic symptoms, but PRV infections in Malaysia, Indonesia, and Vietnam were associated with milder respiratory disease (4,7,12). Because we focused on severe disease, we cannot exclude the possibility that PRVs in Bangladesh can also cause mild infections.
In summary, human PRV infection can have signs and symptoms similar to those of NiV infection. Like NiV infections, PRV infections can be linked to consumption of date palm sap contaminated with bat excreta. The potential for reassortment in segmented viruses like PRV can result in changes in transmissibility and virulence. Thus, in areas where raw date palm sap is consumed, molecular and serologic surveillance and differential diagnoses of respiratory illnesses with encephalitis and other unexplained febrile illnesses should include PRV, NiV, and other batborne viruses.
Dr. Sultana is an assistant professor of virology and currently serves as a senior scientific officer at the Institute of Epidemiology, Disease Control and Research (IEDCR) in Bangladesh. Her research focuses on the intersection of human, animal, and environmental health through the One Health approach. Dr. Islam is a One Health epidemiologist at Charles Sturt University, Wagga Wagga, NSW, Australia. His research interests focus on how viruses spill over from wildlife to humans and how we can integrate viral genetics, epidemiology, ecology, and human behaviour to prevent zoonotic disease transmission.
Acknowledgments
We highly acknowledge laboratory and field staff from CDC, IEDCR, and icddr,b for collecting specimens from clinical cases, recording metadata, and screening for NiV.
This study was approved by the icddr,b research review committee (approval no. PR-2005-026).
This project was supported with funds provided by United States Department of Agriculture agreements with Columbia University (NACA-58-3022-2-021 and NACA-58-3022-4-053).
References
- Letko M, Seifert SN, Olival KJ, Plowright RK, Munster VJ. Bat-borne virus diversity, spillover and emergence. Nat Rev Microbiol. 2020;18:461–71. DOIPubMedGoogle Scholar
- Satter SM, Aquib WR, Sultana S, Sharif AR, Nazneen A, Alam MR, et al. Tackling a global epidemic threat: Nipah surveillance in Bangladesh, 2006-2021. PLoS Negl Trop Dis. 2023;17:
e0011617 . DOIPubMedGoogle Scholar - Bennett AJ, Goldberg TL. Pteropine orthoreovirus in an Angolan soft-furred fruit bat (Lissonycteris angolensis) in Uganda dramatically expands the global distribution of an emerging bat-borne respiratory virus. Viruses. 2020;12:740. DOIPubMedGoogle Scholar
- Tee KK, Chan PQ, Loh AM, Singh S, Teo CH, Iyadorai T, et al. Surveillance, isolation and genomic characterization of Pteropine orthoreovirus of probable bat origin among patients with acute respiratory infection in Malaysia. J Med Virol. 2023;95:
e28520 . DOIPubMedGoogle Scholar - Vega-Rodriguez W, Ly H. Emerging Pteropine orthoreoviruses and their potential impact on public health. J Med Virol. 2023;95:
e28588 . DOIPubMedGoogle Scholar - Gard GP, Marshall ID. Nelson Bay virus. A novel reovirus. Arch Gesamte Virusforsch. 1973;43:34–42. DOIPubMedGoogle Scholar
- Chua KB, Crameri G, Hyatt A, Yu M, Tompang MR, Rosli J, et al. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc Natl Acad Sci U S A. 2007;104:11424–9. DOIPubMedGoogle Scholar
- Kawagishi T, Kanai Y, Tani H, Shimojima M, Saijo M, Matsuura Y, et al. Reverse genetics for fusogenic bat-borne orthoreovirus associated with acute respiratory tract infections in humans: role of outer capsid protein σC in viral replication and pathogenesis. PLoS Pathog. 2016;12:
e1005455 . DOIPubMedGoogle Scholar - Briese T, Kapoor A, Mishra N, Jain K, Kumar A, Jabado OJ, et al. Virome capture sequencing enables sensitive viral diagnosis and comprehensive virome analysis. mBio. 2015;6:e01491–15. DOIPubMedGoogle Scholar
- Harima H, Sasaki M, Orba Y, Okuya K, Qiu Y, Wastika CE, et al. Attenuated infection by a Pteropine orthoreovirus isolated from an Egyptian fruit bat in Zambia. PLoS Negl Trop Dis. 2021;15:
e0009768 . DOIPubMedGoogle Scholar - Lorusso A, Teodori L, Leone A, Marcacci M, Mangone I, Orsini M, et al. A new member of the Pteropine Orthoreovirus species isolated from fruit bats imported to Italy. Infect Genet Evol. 2015;30:55–8. DOIPubMedGoogle Scholar
- Chua KB, Voon K, Crameri G, Tan HS, Rosli J, McEachern JA, et al. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS One. 2008;3:
e3803 . DOIPubMedGoogle Scholar - McDonald SM, Nelson MI, Turner PE, Patton JT. Reassortment in segmented RNA viruses: mechanisms and outcomes. Nat Rev Microbiol. 2016;14:448–60. DOIPubMedGoogle Scholar
- Matthijnssens J, Attoui H, Bányai K, Brussaard CPD, Danthi P, Del Vas M, et al. ICTV virus taxonomy profile: Spinareoviridae 2022. J Gen Virol. 2022;103. DOIGoogle Scholar
- Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, Gurley E, et al. Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis. 2006;12:1888–94. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: December 23, 2025
1These first authors contributed equally to this article.
2These last authors contributed equally to this article.
Table of Contents – Volume 31, Number 12—December 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Nischay Mishra, Mailman School of Public Health, Columbia University, Center for Infection and Immunity, 722 W 168th St, 17th Fl, New York, NY 10032, USA
Top