Volume 31, Number 12—December 2025
Research Letter
Metatranscriptomic Identification of Trubanaman Virus Sequences in Patient with Encephalitis, Australia
Cite This Article
Citation for Media
Abstract
Using metatranscriptomics, we identified Trubanaman virus in cerebrospinal fluid from a severely immunocompromised man who died of encephalitis in Queensland, Australia. Virus sequences were related to orthobunyaviruses previously detected in mosquitoes in Australia. Testing for other causes yielded negative results, suggesting that Trubanaman virus was the cause of this fatal encephalitis case.
Approximately 50% of global encephalitis cases remain undiagnosed by conventional testing (1). Metagenomic next-generation sequencing (mNGS), particularly metatranscriptomics (i.e., total RNA sequencing), is an emerging approach to infection diagnosis that reveals all nucleic acid in a sample, making it ideal for detecting novel and emerging pathogens (2).
Orthobunyavirus (order Bunyavirales) is a diverse genus of negative-sense single-stranded RNA viruses recognized to cause febrile illness and encephalitis in humans globally (3). The best described orthobunyaviruses are La Crosse virus and Jamestown Canyon virus, both of which rarely cause encephalitis, permanent neurologic sequalae, or death (4,5). Jamestown Canyon virus is associated with meningoencephalitis in immunocompromised persons (5), whereas the emerging Oropuche virus is associated with fever, headache, myalgias, and rare cases of meningoencephalitis and has recently expanded its range in Central and South America (6). We used metatranscriptomics to investigate a case of encephalitis in an immunocompromised person in Australia.
The study was approved by the Metro-North Health Human Research Ethics Committee and written informed consent was obtained from the patient and his next of kin. Metatranscriptomic sequencing and analysis methods are detailed (Appendix).
A man in his 50s who lived in West Moreton, Queensland, Australia, was admitted for a volunteer unrelated donor allogeneic hemopoietic stem cell transplantation with posttransplant cyclophosphamide and tacrolimus for B-cell acute lymphoblastic leukemia in complete remission one. There was no central nervous system involvement. He received 8 cycles of rituximab-hyper cyclophosphamide, vincristine, doxorubicin, and dexamethasone before transplantation. The transplant was complicated by a polymicrobial bloodstream infection that was successfully treated with intravenous daptomycin, as well as mucositis and diarrhea.
On day 18 after the hemopoietic stem cell transplantation, the patient experienced fever to 38.8°C, tachycardia to 109 beats/min, muscular pain, intermittent headache, and confusion manifesting as slow and tangential answers to questions, difficulty word-finding, reduced oral intake, disorientation to time and place, and delusions such as thinking that he had been in a car accident. The onset coincided with recovery of his neutrophil and lymphocyte count. His confusion fluctuated but generally deteriorated. Twenty-two days later, a cerebrospinal fluid (CSF) examination was performed (Table). Magnetic resonance imaging (MRI) of the brain was also performed, and results were unremarkable. However, results of an electroencephalograph were abnormal, showing mild, diffuse cortical dysfunction but no epileptiform activity. Results of a nasopharyngeal nucleic acid amplification test (NAAT) were positive for rhinovirus. Stool, blood, and urine culture and NAAT results were negative for viruses, bacteria, and fungi (Table). He was unresponsive to corticosteroids, and during the next few months, his level of consciousness, function, and speech declined; serial MRIs showed progressive cerebral atrophy. He died 6 months after the onset of confusion.
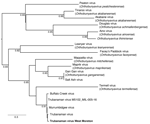
Metatranscriptomic sequencing of the patient’s CSF using NovaSeq (Illumina, https://www.illumina.com) generated a total of 57,452,775 paired reads, of which 74 matched the M glycoprotein precursor of Trubanaman, Murrumbidgee, and Buffalo Creek viruses (i.e., the Mapputta group, which likely represents a single species within the genus Orthobunyavirus). E-values were <10−116. From some of those reads, we assembled a single contig of 270 bp (GenBank accession no. PV702715), denoted Trubanaman virus West Moreton (Figure). We did not recover reads from the RNA-dependent RNA polymerase (RdRp) or other virus genes. The water control was negative for bunyaviruses. Similarly, metatranscriptomic analysis was negative for other known or putative human pathogenic viruses, bacteria, fungi, and parasites, and no other candidate pathogens were identified.
Using metatranscriptomics, we identified Trubanaman virus sequences in a CSF sample from a person with encephalitis. Extensive testing for other infectious, autoimmune, and malignant causes yielded negative results. In the context of a high-risk immunocompromised person with typical clinical manifestations of encephalitis, our findings support, but do not confirm, that Trubanaman virus was the cause of the patient’s encephalitis. PCR could not be performed on the original sample because it was fully depleted for conventional testing and sequencing, although no viable routes to sample contamination existed. Follow-up testing of CSF collected 6 weeks later was negative by both metatranscriptomics and orthobunyavirus-specific PCRs targeting the N protein and RdRp.
Trubanaman and related viruses have been detected in mosquito populations throughout Australia (7). Patients with a suspected arthropodborne virus infection in New South Wales exhibited neutralizing antibody prevalences of 4.7% to Gan Gan virus (GGV) and 1.4% to Trubanaman virus (8). GGV was associated with an acute febrile illness and polyarthritis in 3 persons in Australia who had significant titer rises in paired serum samples, as well as GGV-specific IgM (9). In addition, serologic evidence suggests that kangaroos, feral animals, and domestic horses are reservoirs for orthobunyaviruses in Australia (9). Of note, 2 bunyavirus-associated cases of fatal meningoencephalitis in immunocompromised persons were recently described in the United States using CSF mNGS (10). Further research is required to establish the pathogenic role of Trubanaman virus as a cause of encephalitis in Australia and to determine the arthropod vectors, zoonotic reservoirs, and seroprevalence. However, our findings suggest that Trubanaman virus was the cause of this fatal encephalitis case, and clinicians should be aware of the possibility of infection with this virus in similar cases.
Mr. Hajkowicz is a senior staff specialist and former director of the Infectious Diseases Unit at Royal Brisbane and Women’s Hospital and Clinician Researcher at the University of Queensland Centre for Clinical Research and a PhD student at the University of Sydney School of Medical Sciences. His research interests include detection of emerging viral infections in Australia, including mpox and Zika virus, and pandemic response.
Acknowledgment
We thank Daisy Lindsay for coordinating research ethics and governance and John-Sebastian Eden for assisting with the bioinformatic analysis pipeline.
References
- Schubert RD, Wilson MR. A tale of two approaches: how metagenomics and proteomics are shaping the future of encephalitis diagnostics. Curr Opin Neurol. 2015;28:283–7.PubMedGoogle Scholar
- Elliott RM. Orthobunyaviruses: recent genetic and structural insights. Nat Rev Microbiol. 2014;12:673–85.PubMedGoogle Scholar
- Haddow AD, Odoi A. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States, 2003-2007. PLoS One. 2009;4:
e6145 .PubMedGoogle Scholar - Meier-Stephenson V, Drebot MA, Dimitrova K, DiQuinzio M, Fonseca K, Forrest D, et al. Case series of Jamestown Canyon virus infections with neurologic outcomes, Canada, 2011–2016. Emerg Infect Dis. 2024;30:874–81.PubMedGoogle Scholar
- Riccò M, Corrado S, Bottazzoli M, Marchesi F, Gili R, Bianchi FP, et al. (Re-)emergence of Oropouche virus (OROV) infections: systematic review and meta-analysis of observational studies. Viruses. 2024;16:1498.PubMedGoogle Scholar
- Gauci PJ, McAllister J, Mitchell IR, Weir RP, Melville LF, Gubala AJ. Genomic characterisation of Trubanaman and Gan Gan viruses, two bunyaviruses with potential significance to public health in Australia. Virol Rep. 2016;6:1–10.
- Boughton CR, Hawkes RA, Naim HM. Arbovirus infection in humans in NSW: seroprevalence and pathogenicity of certain Australian bunyaviruses. Aust N Z J Med. 1990;20:51–5.PubMedGoogle Scholar
- Johansen CA, Mackenzie JS, Smith DW, Lindsay MDA. Prevalence of neutralising antibodies to Barmah Forest, Sindbis and Trubanaman viruses in animals and humans in the south-west of Western Australia. Aust J Zool. 2005;53:51–8.
- Chiu CY, Godasi RR, Hughes HR, Servellita V, Foresythe K, Tubati A, et al. Two human cases of fatal meningoencephalitis associated with Potosi and Lone Star virus infections, United States, 2020–2023. Emerg Infect Dis. 2025;31:215–21.PubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: December 24, 2025
Table of Contents – Volume 31, Number 12—December 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Krispin Hajkowicz, School of Medical Sciences, University of Sydney, Biomedical Bldg C81, Central Avenue, Everleigh, NSW 2015, Australia
Top