Effects of Decentralized Sequencing on National Listeria monocytogenes Genomic Surveillance, Australia, 2016–2023
Patiyan Andersson, Sally Dougall, Karolina Mercoulia, Kristy A. Horan, Torsten Seemann, Jake A. Lacey, Tuyet Hoang, Lex E.X. Leong, David Speers, Louise Cooley, Karina Kennedy, Rob Baird, Rikki Graham, Qinning Wang, Avram Levy, Dimitrios Menouhos, Norelle L. Sherry, Susan A. Ballard, Vitali Sintchenko, Amy V. Jennison, and Benjamin P. Howden

Author affiliation: The University of Melbourne, Melbourne, Victoria, Australia (P. Andersson, S. Dougall, K. Mercoulia, K.A. Horan, T. Seeman, J.A. Lacey, T. Hoang, N.L. Sherry, S.A. Ballard, B.P. Howden); SA Pathology, Adelaide, South Australia, Australia (L.E.X. Leong); Queen Elizabeth II Medical Centre, Perth, Western Australia, Australia (D. Speers, A. Levy); Royal Hobart Hospital, Hobart, Tasmania, Australia (L. Cooley); Canberra Health Services, Australian National University Medical School, Canberra, Australian Capital Territory, Australia (K. Kennedy); Territory Pathology, Royal Darwin Hospital, Darwin, Northern Territory, Australia (R. Baird, D. Menouhos); Queensland Public Health and Scientific Services, Queensland Health, Brisbane, Queensland, Australia (R. Graham, A.V. Jennison); Institute of Clinical Pathology and Medical Research, NSW Health Pathology, Sydney, New South Wales, Australia (Q. Wang, V. Sintchenko); Austin Health, Heidelberg, Victoria, Australia (N.L. Sherry, B.P. Howden); Sydney Institute for Infectious Diseases, The University of Sydney, Sydney (V. Sintchenko)
Main Article
Figure 1
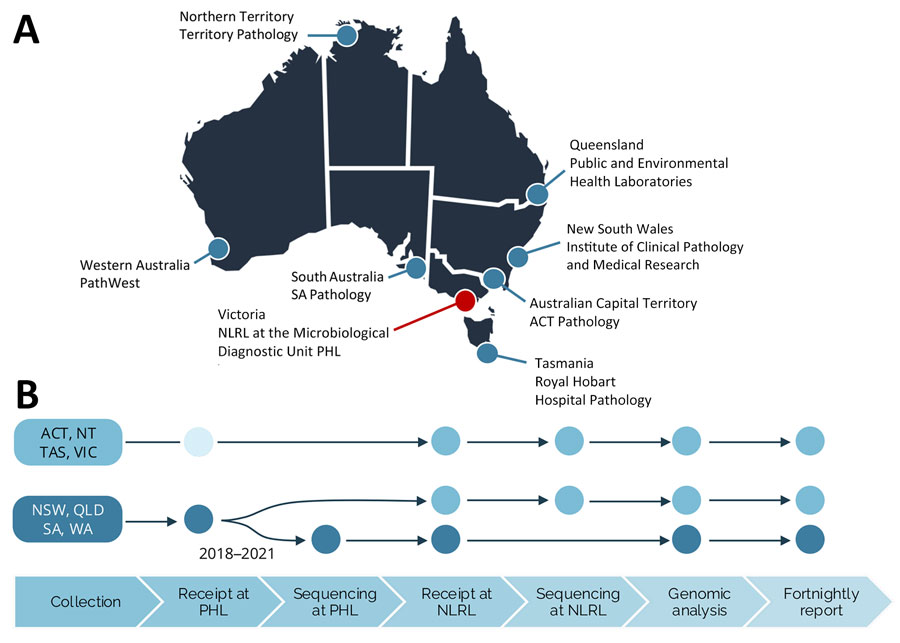
Figure 1. National decentralized sequencing system for Listeria monocytogenes genomic surveillance, Australia. A) Eight jurisdictional public health laboratories contributing to genomic surveillance. The NLRL is based at the Microbiological Diagnostic Unit (MDU) PHL in the state of Victoria. B) Overview of the steps in the national genomic surveillance system; dots indicate where sample processing occurs. The process is the same for human and nonhuman samples. For jurisdictions ACT, NT, TAS, and VIC, sequencing is performed by the NLRL at the Microbiological Diagnostic Unit PHL. The unfilled circle indicates that some samples are referred directly from the primary pathology laboratory to NLRL. For jurisdictions NSW, QLD, SA, and WA, the referral pathway transitioned during 2018–2021 from sequencing performed by the NLRL to jurisdictional sequencing and referral of sequences for genomic analysis. ACT, Australian Capital Territory; NLRL, National Listeria Reference Laboratory; NSW, New South Wales; NT, Northern Territory; PHL, public health laboratory; QLD, Queensland; SA, South Australia; TAS, Tasmania; VIC, Victoria; WA, Western Australia.
Main Article
Page created: April 01, 2025
Page updated: May 12, 2025
Page reviewed: May 12, 2025
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
