Volume 31, Number 2—February 2025
Research
Short-Lived Neutralizing Antibody Responses to Monkeypox Virus in Smallpox Vaccine–Naive Persons after JYNNEOS Vaccination
Figure 2
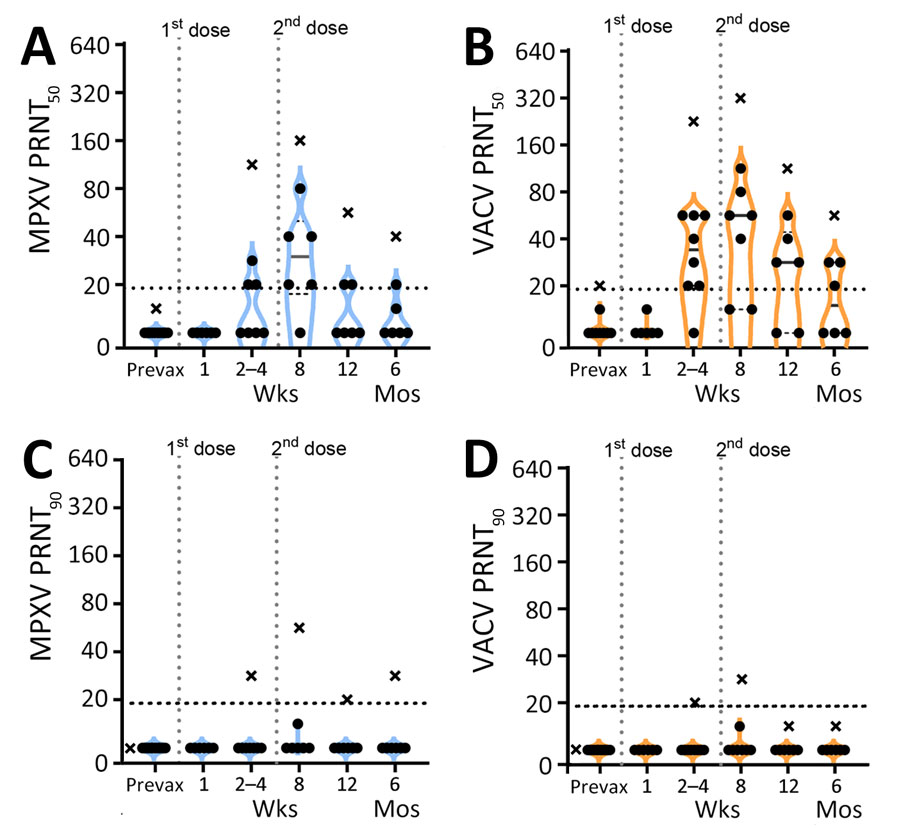
Figure 2. PRNT titers for participants vaccinated with JYNNEOS vaccine up to 6 months prior in a study to assess neutralizing antibody responses to MPXV in smallpox vaccine–naive persons after JYNNEOS vaccination. We used PRNT to test serum samples from donors vaccinated with 2 doses of JYNNEOS vaccine ≈28 days apart. We performed assays with sonicated virus and a 1-hour virus–serum incubation. A) MPXV PRNT50 results; B) VACV PRNT50 results; C) MPXV PRNT90 results; D) VACV PRNT90 results. Participants with no known vaccinia exposure (black circles) are used for mean calculations. Data from a single donor with prior smallpox vaccination (black Xs) are plotted separately and excluded from mean calculations. Each datapoint represents the geometric mean titer of 2 independent experiments. The vertical dotted lines represent the timing of the vaccine doses, and the horizontal dotted lines indicate limits of detection. MPXV, monkeypox virus; prevax, prevaccination; PRNT, plaque reduction neutralization test; PRNT50, 50% plaque reduction as measured by PRNT; PRNT90, 90% plaque reduction as measured by PRNT; VACV, vaccinia virus.