Volume 31, Number 4—April 2025
Research Letter
Local Circulation of Sindbis Virus in Wild Birds and Horses, the Netherlands, 2021–2022
Abstract
We report Sindbis virus circulation in the Netherlands based on serologic evidence found in 6 resident wild birds and 3 horses (2021–2022). Tested mosquitoes were molecularly negative, and humans were serologically negative. Veterinarians and health practitioners in the Netherlands should be aware of the importance of surveillance for Sindbis virus.
Sindbis virus (SINV; family Togaviridae, genus Alphavirus) is maintained in an enzootic transmission cycle between birds (e.g., passerines and grouse) and mosquito vectors (mainly Culex spp., but also Aedes and Culiseta spp.) (1). Horses and humans are considered dead-end hosts. Clinical cases in humans are commonly reported in northern Europe (Finland and Sweden) and South Africa (2,3). Studies have described nonsymptomatic infections in horses in Sweden through the detection of neutralizing antibodies (4), but neurologic signs can also develop in horses, as shown in South Africa (5). Many countries in Europe have reported evidence of exposure in wildlife, including Germany and the United Kingdom, but not the Netherlands (2). Therefore, we investigated the presence of SINV in the Netherlands in mosquitoes, birds, horses, and humans.
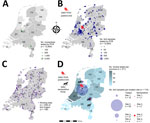
Throughout the Netherlands, we screened mosquitoes, wild birds, horses, and humans for SINV RNA and neutralizing antibodies (Figure; Appendix Figure 1). We sampled mosquitoes (n = 12,884) from July to mid-October in 2020, 2021, and 2022 as previously described (6) and live wild birds (n = 10,983) in 2021 and 2022 as part of ongoing research into the arbovirus dynamics in birds (7). We collected serum samples from horses (n = 368) from May 2021 through 2022 (8) and additional serum samples (n = 3) in October 2023. We also collected serum samples from bird ringers (n = 148), which are actively ringing birds and may be at higher risk for infection, from June to September 2021 as previously described (9).
We used real-time reverse transcription PCR (RT-PCR) to test all mosquitoes and birds for SINV RNA (Appendix). All mosquito pools tested negative (Figure, panel A; Appendix Figure 2, panel A). Of the live bird samples screened (9,599 birds caught/tested once and 593 birds caught/tested repeatedly), we detected SINV RNA (pooled from swab and feather samples) in 1 adult European robin (Erithacus rubecula) caught in Naarden, Noord-Holland (October 23, 2022) (Figure, panel B; Appendix Figure 2, panels B, C). A partial SINV sequence from that bird clustered with SINV genotype I sequences from Germany, Nordic countries (Finland, Sweden, and Norway), and Russia (99% identity to all) (Appendix Figure 3). The partial SINV sequence was deposited in GenBank (accession no. PQ215107).
We initially tested a total of 368 horses for SINV neutralizing antibodies by using a plaque reduction neutralization test (PRNT) based on >80% plaque reduction (Appendix Figure 4) (10). Three (0.82%, 95% CI 0.28%–2.37%) of 368 mares sampled during July–December 2021 were seropositive, and endpoint titers ranged from 20 to 80 (Table; Figure, panel D). None of the horses traveled outside of the Netherlands. We examined serum samples of wild birds trapped within a 16-km radius of the 3 seropositive horses and the RT-PCR–positive bird by using PRNT (2021–2022; n = 110); 12 (10.91%, 95% CI 6.35%–18.10%) of those bird serum samples were seropositive (Table; Figure, panel D). Of the 110 bird samples tested, we derived 4 samples from 2 birds (identification no. L521438, positive and later negative; identification no. ID L413285, twice negative; Table). We found neutralizing antibodies in common blackbirds (Turdus merula; n = 10) and song thrushes (Turdus philomelos; n = 2) from the Turdidae family (Appendix Figure 5). The titers varied from 20 to 320 (Table). All the 148 human bird ringer serum samples tested were SINV seronegative (Figure, panel C).
Our findings verify the circulation of SINV in the Netherlands by detection of RNA in a European robin and seropositive common blackbirds, song thrushes, and horses in distinct geographic regions. Seropositive birds caught in May and June (2 song thrushes and 3 blackbirds) and 1 bird caught multiple times within 1 season (1 blackbird) are considered resident breeding birds in the Netherlands and likely acquired an infection with SINV locally. The detection of neutralizing antibodies in horses without travel history and resident birds in the Netherlands does strongly indicate locally acquired infections. However, additional isolation of SINV (whole-genome) sequences from infected mosquitoes, animals, or humans in the Netherlands is required to make conclusions over the exact route of SINV introduction.
This study did not detect SINV seropositivity in humans, possibly because of the low sample size in this study. No human SINV infections have been reported thus far in the Netherlands. However, the lack of reported human SINV infections might be either caused by limited awareness and, therefore, no testing or absence of spillovers into the human population. Detailed studies are needed to assess possible human prevalence. Future studies should target screening of mosquitoes, birds, horses, and humans at the end of the mosquito season in the same areas where seropositive cases were detected thus far. Meanwhile, veterinarians and health practitioners in the Netherlands should increase their awareness regarding surveillance for SINV and potential risk to humans from its circulation.
Dr. Streng is a veterinarian and a graduate of the Wageningen University & Research, where she studies (mosquitoborne) arboviruses in domestic and wild animals as part of a research consortium One Health PACT (Predicting Arbovirus Climate Tipping points). Dr. Holicki is a veterinarian and postdoctoral researcher at the Erasmus Medical Center in Rotterdam, and her research focuses on the surveillance and pathogenicity of (re-)emerging arboviruses in the Netherlands.
Acknowledgments
We thank Irina V. Chestakova, Marjan Boter, Isa Meeuwisse, Babette Weller, and Merve Tok for their contributions to sample processing and testing and Tijs van den Berg, Natasja van Nijen, Jurrian van Irsel, Louella M.R. Kasbergen, and Patricia C.J.L. Bruijning-Verhagen for assistance in sample and metadata collection for the birds and bird ringers. Furthermore, we thank all horse owners and veterinarians for their cooperation in this study, as well as all bird ringers for their collection of bird and mosquito specimens.
Horses in this study were sampled under legislation (license no. AVD40100202114384) of the Dutch Central Authority for Scientific procedures on Animals. Sampled birds were ringed, and sampling was performed under ethical permit nos. AVD801002015342 and AVD80100202114410 issued to Netherlands Institute of Ecology. The sampling of the bird ringers was approved by the Medical Research Ethics Committee of Leiden, The Hague, Delft, the Netherlands (approval no. P20.112).
This work was supported by the Dutch Research Council (project “Preparing for vector-borne virus outbreaks in a changing world: a One Health Approach”; NWA.1160.1S.210), the European Union’s Horizon 2020 research and innovation programme (grant no. 874735), and the Health Emergency Preparedness and Response and the European-Union’s EU4Health programme (grant no. 101102733).
References
- Adouchief S, Smura T, Sane J, Vapalahti O, Kurkela S. Sindbis virus as a human pathogen-epidemiology, clinical picture and pathogenesis. Rev Med Virol. 2016;26:221–41. DOIPubMedGoogle Scholar
- European Center for Disease Prevention and Control. Facts about Sindbis fever [cited 2024 Jun 7]. https://www.ecdc.europa.eu/en/sindbis-fever/facts
- Storm N, Weyer J, Markotter W, Kemp A, Leman PA, Dermaux-Msimang V, et al. Human cases of Sindbis fever in South Africa, 2006-2010. Epidemiol Infect. 2014;142:234–8. DOIPubMedGoogle Scholar
- Björnström A, Blomström AL, Singh MC, Hesson JC. Sindbis virus neutralising antibodies detected in Swedish horses. One Health. 2021;12:
100242 . DOIPubMedGoogle Scholar - Fourie I, Snyman J, Williams J, Ismail A, Jansen van Vuren P, Venter M. Epidemiological and genomic characterisation of Middelburg and Sindbis alphaviruses identified in horses with febrile and neurological infections, South Africa (2014–2018). Viruses. 2022;14:2013. DOIPubMedGoogle Scholar
- Krol L, Blom R, Dellar M, van der Beek JG, Stroo ACJ, van Bodegom PM, et al. Interactive effects of climate, land use and soil type on Culex pipiens/torrentium abundance. One Health. 2023;17:
100589 . DOIPubMedGoogle Scholar - Sikkema RS, Schrama M, van den Berg T, Morren J, Munger E, Krol L, et al. Detection of West Nile virus in a common whitethroat (Curruca communis) and Culex mosquitoes in the Netherlands, 2020. Euro Surveill. 2020;25:
2001704 . DOIPubMedGoogle Scholar - Streng K, Hakze-van der Honing RW, Graham H, van Oort S, de Best PA, Abourashed A, et al. Orthoflavivirus surveillance in the Netherlands: Insights from a serosurvey in horses & dogs and a questionnaire among horse owners. Zoonoses Public Health. 2024;71:900–10. DOIPubMedGoogle Scholar
- de Bellegarde de Saint Lary C, Kasbergen LMR, Bruijning-Verhagen PCJL, van der Jeugd H, Chandler F, Hogema BM, et al. Assessing West Nile virus (WNV) and Usutu virus (USUV) exposure in bird ringers in the Netherlands: a high-risk group for WNV and USUV infection? One Health. 2023;16:
100533 . DOIPubMedGoogle Scholar - Hesson JC, Lundström JO, Tok A, Östman Ö, Lundkvist Å. Temporal variation in Sindbis virus antibody prevalence in bird hosts in an endemic area in Sweden. PLoS One. 2016;11:
e0162005 . DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: March 13, 2025
1These first authors contributed equally to this article.
2These senior authors contributed equally to this article.
Table of Contents – Volume 31, Number 4—April 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Reina S. Sikkema, Viroscience, Erasmus Medical Center, Dr. Molewaterplein 40, 3015 GD, Rotterdam, the Netherlands
Top