Volume 31, Number 5—May 2025
Research Letter
Unexpected Zoonotic and Hybrid Schistosome Egg Excretion Patterns, Malawi, 2024
Abstract
Two exemplary cases of mixed urogenital and intestinal schistosomiasis in Malawi show hybridizations of Schistosoma mattheei with S. haematobium and S. mansoni, indicating newly emerging genetic diversity. Complex egg excretion patterns in feces expose current diagnostic gaps and alert to future sampling needs for effective surveillance of zoonotic schistosomiasis.
Schistosomiasis is a waterborne, parasitic disease transmitted by several species of Bulinus and Biomphalaria, two distinct freshwater snail genera common across sub-Saharan Africa (1). In sub-Saharan Africa, Schistosoma haematobium is the predominant cause of urogenital schistosomiasis, and S. mansoni is the predominant cause of intestinal schistosomiasis (1). S. haematobium is endemic in Malawi, where infections with zoonotic and hybrid species from the S. haematobium group (S. mattheei and S. haematobium × S. mattheei) have also been detected in humans (2,3). S. mattheei is considered a livestock-infecting schistosome that causes intestinal disease (4); however, excretion of ova from humans infected with S. mattheei and associated S. haematobium group hybrids reportedly has occurred through the urogenital tract (2,3). Meanwhile, Biomphalaria freshwater snails were first detected along the southern shores of Lake Malawi in 2017 (5). Since then, autochthonous S. mansoni transmission and intestinal schistosomiasis outbreaks have been confirmed in Mangochi District, Malawi (5,6).
To clarify S. haematobium group hybridization dynamics, we conducted a longitudinal cohort study in southern Malawi. The College of Medicine Research Ethics Committee, Malawi (approval no. P.08/21/3381, https://www.ncst.mw) and the Liverpool School of Tropical Medicine Research Ethics Committee, United Kingdom (approval no. 22-028, https://www.lstmed.ac.uk/research/research-integrity/research-ethics-committee) provided ethics approval. This study also tracked S. mansoni prevalence in a community cohort recruited from Samama Village, Mangochi District (Appendix Figure 1), where the outbreak of intestinal schistosomiasis was initially reported (5,6).
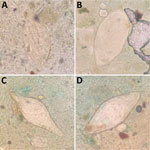
In June 2024, we determined S. mansoni prevalence in Mangochi District to be 14.8% (165/1,116) using point-of-care urine circulating cathodic antigen cassette tests (POC-CCA; ICT International, https://ictinternational.com), and considered trace results positive. Those results represented the lowest reported S. mansoni prevalence in Samama Village since it emerged in 2017 (5–7). However, we observed numerous atypical schistosome ova within feces provided by 2 POC-CCA–positive participants upon Kato-Katz examination (https://microbeonline.com/kato-katz-technique-principle-procedure-results) (Figure). Patient X, a 10-year-old girl, and patient Y, a 19-year-old man (Table), both received treatment with praziquantel from a study-affiliated clinician. The unexpected morphologic diversity raised concerns about underreporting of intestinal schistosomiasis being caused by species other than S. mansoni, prompting closer molecular analysis for robust speciation that cannot be achieved by microscopy.
We obtained hatched miracidia from the urine and feces of patients X and Y by using Pitchford-Visser filtration and collected and preserved individual miracidia on Whatman Flinders Technology Associates cards (GE Healthcare Life Sciences, https://www.gehealthcare.com/products/life-sciences), according to standard protocols (8). To identify the schistosome larvae, we used a newly described 2-tube high-resolution melt real-time PCR assay on DNA extracted from individual preserved miracidia (9). To detect evidence of mixed ancestry or putative genetic introgression, we targeted both the nuclear DNA ribosomal internal transcribed spacer 2 locus and species-specific mitochondrial DNA (mtDNA) loci of 6 Schistosoma species: S. bovis, S. curassoni, S. haematobium, S. mansoni, S. margrebowiei, and S. mattheei. For S. bovis, S. curassoni, S. haematobium, and S. mansoni we targeted the tRNA lysine region; for S. margrebowiei the NADH dehydrogenase subunit 4 region; and for S. mattheei, the NADH dehydrogenase subunit 6 region.
Miracidia hatched from ova in the feces of patient X mostly typed as S. haematobium × S. mattheei hybrids (93.3%), whereas most miracidia hatched from the paired urine sample typed as pure S. haematobium (95.8%). Similarly, atypical zoonotic and hybrid schistosome species ova from patient Y were predominantly in feces (Appendix Figure 2). Of the 59 S. haematobium × S. mattheei hybrid miracidia typed from feces, high-resolution melt profiles indicated that mtDNA (maternal) was inherited from S. mattheei in 58 miracidia, although S. haematobium mtDNA was detected in the remaining S. haematobium × S. mattheei miracidia. From patient Y, 1 miracidium showed mixed ancestry between S. mansoni and S. mattheei, with discordance between mtDNA and nuclear DNA profiles (Appendix Figure 3). Although adult worm pairings between distantly related species usually result in the production of parthenogenetic eggs, previous experimental pairings of S. mansoni and S. mattheei resulted in the production of eggs viable to the third generation (10).
Our findings not only provide evidence of complex hybridization events in the natural setting among S. haematobium, S. mansoni, and S. mattheei but also highlight the greater relative abundance of zoonotic and hybrid schistosome species ova in feces compared with paired urine samples. That observation suggests that S. mattheei and associated hybrids, previously linked to urogenital schistosomiasis in humans, may dominate in intestinal infections by migrating to the intestinal mesenteries, just as S. mattheei likely does in other mammalian hosts where it causes rectal schistosomiasis (4). POC-CCA tests were not designed to detect zoonotic schistosomiasis; although we acknowledge that patients X and Y were both POC-CCA positive, those results do not assure the ability of POC-CCA tests to detect S. haematobium group intestinal infections because we did not perform detailed inspection of feces from POC-CCA–negative participants in the field.
In summary, we detected S. haematobium × S. mattheei hybrid ova from 2 patients in Malawi. Further fecal sampling and molecular testing with species-specific TaqMan probe assays will be essential for monitoring intestinal schistosomiasis in coendemic areas where zoonotic transmission could occur.
Dr. O’Ferrall is a clinician-scientist and PhD researcher in the Department of Tropical Disease Biology at the Liverpool School of Tropical Medicine, UK. His research focuses on the genomic epidemiology of pathogens in tropical settings, including drug-resistant bacteria and schistosomes.
Acknowledgments
We thank all participants in our study, particularly the 2 participants providing samples that enabled this analysis. We are grateful to staff and students at the Liverpool School of Tropical Medicine, Malawi-Liverpool Wellcome Programme, and Mangochi District Hospital for their valuable support and expertise in the study, including Alexandra Juhász, Sam Jones, Gladys Namacha, David Lally Jr., Priscilla Chammudzi, Donales R. Kapira, Bright Mainga, Clinton Nkolokosa, Ruth Cowlishaw, Sarah Rollason, and James E. LaCourse.
This research was funded by the National Institute for Health Research and Wellcome Trust through a Joint Investigator Award (grant no. WT 220818/Z/20/Z). A.M. O’Ferrall is supported by the Medical Research Council via the Liverpool School of Tropical Medicine-Lancaster University doctoral training partnership (grant no. MR/W007037/1).
References
- Buonfrate D, Ferrari TCA, Akim Adegnika A, Russell Stothard J, Gobbi FG. Human schistosomiasis. Lancet. 2025;405:658–70. DOIPubMedGoogle Scholar
- Webster BL, Alharbi MH, Kayuni S, Makaula P, Halstead F, Christiansen R, et al. Schistosome interactions within the Schistosoma haematobium group, Malawi. Emerg Infect Dis. 2019;25:1245–7. DOIPubMedGoogle Scholar
- Kayuni S, Cunningham L, Mainga B, Kumwenda D, Jnr DL, Chammudzi P, et al. Detection of male genital schistosomiasis (MGS) associated with human, zoonotic and hybrid schistosomes in Southern Malawi. BMC Infect Dis. 2024;24:839. DOIPubMedGoogle Scholar
- Juhász A, Makaula P, Cunningham LJ, Jones S, Archer J, Lally D Jr, et al. Revealing bovine schistosomiasis in Malawi: Connecting human and hybrid schistosomes within cattle. One Health. 2024;19:
100761 . DOIPubMedGoogle Scholar - Alharbi MH, Condemine C, Christiansen R, LaCourse EJ, Makaula P, Stanton MC, et al. Biomphalaria pfeifferi snails and intestinal schistosomiasis, Lake Malawi, Africa, 2017–2018. Emerg Infect Dis. 2019;25:613–5. DOIPubMedGoogle Scholar
- Kayuni SA, O’Ferrall AM, Baxter H, Hesketh J, Mainga B, Lally D Jr, et al. An outbreak of intestinal schistosomiasis, alongside increasing urogenital schistosomiasis prevalence, in primary school children on the shoreline of Lake Malawi, Mangochi District, Malawi. Infect Dis Poverty. 2020;9:121. DOIPubMedGoogle Scholar
- Archer J, Cunningham LJ, Juhász A, Jones S, O’Ferrall AM, Rollason S, et al. Molecular epidemiology and population genetics of Schistosoma mansoni infecting school-aged children situated along the southern shoreline of Lake Malawi, Malawi. PLoS Negl Trop Dis. 2024;18:
e0012504 . DOIPubMedGoogle Scholar - Stothard JR, Webster BL, Weber T, Nyakaana S, Webster JP, Kazibwe F, et al. Molecular epidemiology of Schistosoma mansoni in Uganda: DNA barcoding reveals substantial genetic diversity within Lake Albert and Lake Victoria populations. Parasitology. 2009;136:1813–24. DOIPubMedGoogle Scholar
- Cunningham LJ, Kayuni S, Juhász A, Makaula P, Lally D Jr, Namacha G, et al. A rapid DNA screening method using high-resolution melt analysis to detect putative Schistosoma haematobium and Schistosoma mattheei hybrids alongside other introgressing schistosomes. Front Trop Dis. 2024;5:
1350680 . DOIGoogle Scholar - Leger E, Webster JP. Hybridizations within the Genus Schistosoma: implications for evolution, epidemiology and control. Parasitology. 2017;144:65–80. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: April 17, 2025
1These senior authors contributed equally to this article.
Table of Contents – Volume 31, Number 5—May 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Russell Stothard, Department of Tropical Disease Biology, Liverpool School of Tropical Medicine, Pembroke Place, Liverpool L3 5QA, UK
Top