Volume 31, Number 8—August 2025
Research Letter
Seroprevalence of Rift Valley and Crimean-Congo Hemorrhagic Fever Viruses, Benin, 2022–2023
Figure 1
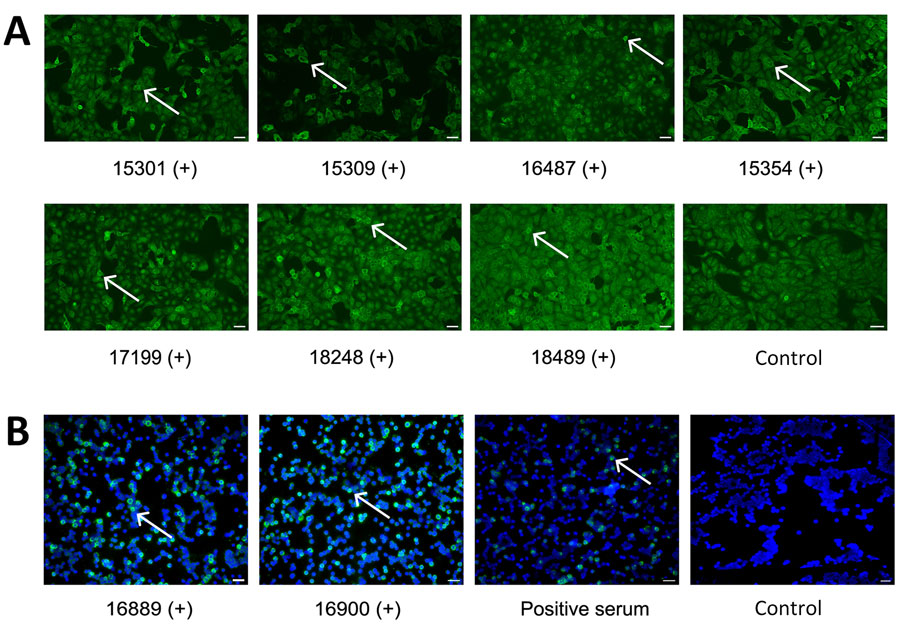
Figure 1. IFA for IgG against Rift Valley and Crimean-Congo hemorrhagic fever viruses, Benin, 2022–2023. A) Serum samples were tested using a commercial IFA (Euroimmun, https://www.euroimmun.com) with Rift Valley fever virus–infected Vero cells. Positive samples are shown at 1:100 dilution; white arrows mark infected cells. B) Serum samples were tested using in-house IFA with Crimean-Congo hemorrhagic fever virus–infected Vero cells (Appendix). Positive samples are shown at 1:10 dilution; white arrows mark infected cells. Titers are provided for the individual samples (Appendix Table). Noninfected controls are shown. Scale bars indicate 20 μm. +, positive serum sample; IFA, immunofluorescence assay.
Page created: June 23, 2025
Page updated: August 18, 2025
Page reviewed: August 18, 2025
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.