Volume 31, Number 9—September 2025
Perspective
Chagas Disease, an Endemic Disease in the United States
Abstract
Chagas disease, caused by Trypanosoma cruzi parasites, is considered endemic to 21 countries in the Americas, excluding the United States. However, increasing evidence of T. cruzi parasites in the United States in triatomine insects, domestic animals, wildlife, and humans challenges that nonendemic label. Several triatomine species are common in the southern United States, where they transmit T. cruzi and invade human dwellings. Wildlife, captive animals, and companion animals, especially dogs, are commonly infected with T. cruzi parasites in this region and serve as reservoirs. Autochthonous human cases have been reported in 8 states, most notably in Texas. Labeling the United States as non–Chagas disease–endemic perpetuates low awareness and underreporting. Classification of Chagas disease as endemic, in particular as hypoendemic, to the United States could improve surveillance, research, and public health responses. Acknowledging the endemicity of Chagas disease in the United States is crucial for achieving global health goals.
Chagas disease, or American trypanosomiasis, is caused by the parasitic protozoan Trypanosoma cruzi, which is transmitted through congenital, oral, and vectorborne routes; vectorborne infections result from contact with the feces of infected triatomine insects (kissing bugs). The World Health Organization (WHO) and Pan American Health Organization highlight 21 countries in the Americas to which Chagas disease is endemic (https://www.who.int/publications/i/item/9789240010352; https://www.paho.org/en/topics/chagas-disease), excluding the United States. As a result, the United States is often labeled as nonendemic, and this designation permeates the scientific literature (1,2), the Centers for Disease Control and Prevention (CDC) website (https://www.cdc.gov/chagas/index.html), the media, pest professional websites, and the general community of researchers and physicians (3,4). In this article, we review a body of evidence establishing the robust presence of T. cruzi parasites in the United States, not only among insect vectors, wildlife, and domestic animals but also among humans without travel histories who are assumed to be locally infected. Through revisiting definitions of endemicity, we conclude that sufficient evidence exists to support the inclusion of the United States as an endemic country for Chagas disease.
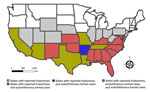
In the United States, triatomines are commonly known as kissing bugs. The blood-sucking insects occur naturally in the southern half of the country and have been identified in 32 states (https://www.cdc.gov/chagas/index.html) (5) (Figure 1). Although available data are inadequate to prove that triatomines are increasing in geographic distribution or abundance, largely owing to a lack of standardized surveillance over time, triatomines are increasingly recognized because of frequent encounters with humans in the domestic and peridomestic habitat and increased research attention (6). Invasion into homes, human bites, subsequent allergic reactions or exposure to T. cruzi parasites, and increasing frequency of canine diagnoses have led to growing public awareness (7–10). Of all 11 triatomine species found in the United States, 9 have been found to be naturally infected with T. cruzi (9,11). Of those, 4 species (Triatoma sanguisuga, T. gerstaeckeri, T. protracta, and T. rubida) are commonly found in human dwellings, raising concern for increased opportunity for vectorborne transmission to humans (7,8,12,13). Although triatomine colonization (defined as the presence of flightless immature nymphal stages in the domicile) occurs in the United States (12,14), metrics of colonization are lower than those observed in Chagas disease–endemic communities in rural Latin America. Numerous investigations of triatomines in the United States have revealed they harbor T. cruzi parasites; infection prevalence ranges from 30% to >50% (15,16). Triatoma sanguisuga and T. protracta kissing bugs have the largest overall distribution within the United States, but the T. gerstaeckeri kissing bug appears to be the most common species in domestic settings in Texas and is likely responsible for transmission resulting in locally acquired T. cruzi infection in dogs and humans (9).
T. cruzi infections among sylvatic and peridomestic mammalian reservoirs have been documented in >17 states in the southern United States (Figure 1) (Appendix) and include species such as woodrat (Neotoma spp.), Virginia opossum (Didelphis virginiana), raccoon (Procyon lotor), nine-banded armadillo (Dasypus novemcinctus), striped skunk (Mephitis mephitis), and coyote (Canis latrans) (6). Infection prevalence among some wild mammal populations can be as high as >50%, and parasitemias are considered high enough to infect triatomines, thus these mammals function as reservoir hosts (6,17). T. cruzi discrete typing units I and IV have been consistently identified in wild and domestic reservoir species (6,9,18); additional discrete typing units have been detected using deep-sequencing methods (19). Among wildlife reservoirs in the United States, Virginia opossums can possess the unique feature of harboring T. cruzi parasites within the anal gland and anal gland secretions, and vertical transmission from infected mother opossum to joey has been shown (19,20); those observations suggest alternative parasite transmission pathways within wildlife.
Infection among companion animals, such as domestic and working canines and felines, has also been demonstrated throughout the United States (6). Dogs exposed to T. cruzi have been found in 23 states, as well as in Washington, DC, and the US Virgin Islands, although dogs infected in northern states likely reflect travel from regions where vectors are present (16,20). In Texas, the only state where Chagas disease in animals has been a reportable condition, 431 canine cases were reported during 2013–2015 (in addition to cases in 2 cats, 1 horse, 1 rat, 3 chimpanzees, and 1 walrus) (https://www.dshs.texas.gov/notifiable-conditions/zoonosis-control/zoonosis-control-diseases-and-conditions/chagas-disease/chagas-disease-data). After that period of widespread documenting of canine infections, the reporting requirement ceased, in part because of the substantial resources required to collate reports. Canine Chagas disease has been most studied in Texas, where cross-sectional and cohort studies have shown a prevalence ranging from ≈10% to >50% and a study across several large dog kennels showed an incidence of 30.7 new infections/100 dogs/year (e.g., 21,22). Canines are a major domestic reservoir of T. cruzi parasites among communities in Latin America where human infection is routinely demonstrated (23). That link has also been shown in Texas communities located along the Rio Grande River, where infected canines and humans have been documented, raising additional concerns regarding ongoing domestic T. cruzi transmission (24–27).
T. cruzi infection among zoo-housed, exotic mammals has been recognized in states known to have triatomines, including Georgia, Alabama, Kansas, and Texas (6). In addition, infections occur in nonhuman primates at biomedical research facilities across the southern United States, posing challenges for research with those animal models (28). Exact transmission routes to these animals are hard to determine, but transmission likely occurs by ingesting the triatomine bug (29). Although many exotic animals have extensive travel histories, local infections are possible when triatomine are present on the premises.
Autochthonous human T. cruzi infections have been identified in 8 states: California, Arizona, Texas, Tennessee, Louisiana, Missouri, Mississippi, and Arkansas (8). A systematic literature review found 29 confirmed and 47 suspected cases of locally acquired Chagas disease during 2000–2018; shared risk factors included rural residence, history of hunting or camping, and agricultural or outdoor work (30). Those numbers likely greatly underrepresent underlying human infections. The Council of State and Territorial Epidemiologists created a surveillance case definition for T. cruzi infection and Chagas disease in June 2024 (31); however, human Chagas disease is not a nationally notifiable disease, and thus the true prevalence or incidence of autochthonous Chagas disease remains unknown. Human Chagas disease is a notifiable disease in 8 states (Arizona, Arkansas, Louisiana, Mississippi, Tennessee, Texas, Utah, and Washington) and 2 California health jurisdictions (San Diego County and Los Angeles County).
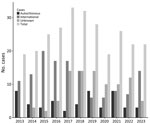
Texas has undertaken extensive efforts to document human Chagas disease; cases were first made reportable in the state in 2013 (https://www.dshs.texas.gov/notifiable-conditions/zoonosis-control/zoonosis-control-diseases-and-conditions/chagas-disease/chagas-disease-data). The first known autochthonous case of human Chagas disease in the United States occurred in an infant in 1955 in Corpus Christi, Texas, in a home known to be infested with triatomines (32). However, parasite transmission to humans in the region has occurred since prehistoric times, given, for example, a paleoparasitology study that recovered T. cruzi DNA in a mummified body (dated to 1,150 BP) of a man from western Texas with signs of megacolon (33). During 2013–2023, the Texas Department of State Health Services documented 50 probable and confirmed cases that were considered autochthonous, either because the area of vector exposure was known or because of a lack of travel to or previous residence in Chagas disease–endemic areas of Latin America (https://www.dshs.texas.gov/notifiable-conditions/zoonosis-control/zoonosis-control-diseases-and-conditions/chagas-disease/chagas-disease-data) (Figure 2; Appendix). Of those 50 cases, 3 were diagnosed at the acute stage, 44 at the chronic asymptomatic (indeterminate) stage, and 3 at the chronic symptomatic (determinate) stage. Based on CDC guidelines, diagnosis of chronic Chagas disease requires positive results by >2 tests that detect antibodies to different antigens, because no single test is sufficiently sensitive and specific for diagnosis (https://www.cdc.gov/chagas/index.html). Of the 47 reported chronic cases, 31 (66%) were confirmed with serologic testing at CDC. Of the 3 acute cases, 2 were acquired in central Texas (Austin–Round Rock metropolitan area); the third case was acquired in the Rio Grande Valley region of South Texas. A Spearman correlation test indicated that there was no temporal trend in the reported cases by year (z-score −1.0004; p = 0.31), underscoring that locally acquired cases are a stable threat in the state.
Triatomine species from the southwestern United States have been shown to have a longer postfeeding defecation behavior than more efficient triatomine vectors in Latin America (34); that behavior has been posed to reduce the risk for contact between infectious T. cruzi and the host. That narrative has contributed to the perception that triatomine species in the United States are not capable of stercorarian transmission. However, a study of T. gerstaeckeri and T. sanguisuga bugs documented simultaneous feeding and defecation by the 2 key North American vectors, although the measured postfeeding defecation indices were longer than those of the Rhodnius Prolixus kissing bug, a highly competent triatomine from South America (35). Although many of the autochthonous Chagas disease cases in the United States involve persons exposed to triatomines, additional case-patients report no exposure to triatomines (36,37), underscoring the cryptic nature of the vectors and suggesting alternative transmission routes should be considered (8).
Oral transmission has been documented in certain regions of Latin America where fruit-based food or drink products have been contaminated with T. cruzi from triatomines living on trees (38). Triatomines in the United States have not been reported to inhabit fruit trees, and oral transmission is likely less relevant for explaining human cases acquired in the United States; nonetheless, oral parasite transmission through consumption of infected triatomines is speculated to be the primary mode of transmission to dogs (39). In addition, screening programs to detect potential congenital T. cruzi transmission in the United States are critical for preventing long-term sequelae of the disease (40).
The CDC defines endemic as the constant presence/usual prevalence of a disease or infectious agent in a population within a geographic area (41). In its characterization of infectious disease occurrence patterns, Clay’s Handbook of Environmental Health identifies an endemic pattern as “when an infection is always present at low or moderate levels within a given geographic area or defined population” (42). A “hyperendemic pattern,” the authors add, “is observed when infection occurs at high levels and affects all age groups equally” (42). WHO provides a more nuanced definition of specific terminology for malaria, such as endemic being “an area in which there is an ongoing, measurable incidence of malaria infection and mosquito-borne transmission over a succession of years”; the organization also includes specific subcategories for the percent of the population with malaria: “hypoendemic (0–10%), mesoendemic (10–50%), hyperendemic (constantly >50%), and holoendemic (constantly >75%)” (43). Such an operative scale for Chagas disease has not yet been developed, but acknowledging the nuanced nature of endemicity (as stated by WHO terminology) and the unique characteristics of Chagas disease occurrence in different regions will be crucial when considering the endemicity status of Chagas disease in the United States. The context in the southern United States presents well-established enzootic cycles and sporadic albeit constant locally acquired human cases (Figure 2), supporting a Chagas disease–endemic disease status.
The current classification of the United States as nonendemic for Chagas disease has led to critical issues such as low physician and veterinary awareness of possible human and animal exposure to T. cruzi (4,44,45), which prevents appropriate differential diagnosis and could subsequently contribute to potential underreporting. The nonendemic label is coupled with the portrayal of Chagas disease as an essentially foreign or an exclusively travel-related issue in the media. Such misrecognition impedes effective disease management and underscores the need to reevaluate Chagas disease’s endemic status.
We propose that Chagas disease in the United States be classified as endemic and, more specifically, hypoendemic, acknowledging its presence and effects while emphasizing the need for heightened awareness and surveillance. We recognize the burden of locally acquired human disease in the United States does not approach the levels seen in some regions of Latin America but hope that labeling the United States as Chagas disease–endemic will also raise awareness for this neglected disease across its endemic range. This reclassification reflects a broader understanding of epidemiology that aligns with a One Health approach, recognizing the interconnectedness of human, animal, and environmental health. It also acknowledges the United States’ foundational and ongoing dependence on the highly variable modes of human migration and settlement. By incorporating ecologic, social, and geographic relationships, this shift paves the way for expanding research and intervention strategies. The United States contributes 23.3% of the world’s scientific research on Chagas disease, but most of it is focused on pharmacological and diagnostics development and immunology (46). Social and epidemiologic research, which should focus on populations that are disproportionately affected (46), is lacking. Recognizing Chagas disease as endemic to the United States would ideally help increase funding agencies’ investment in research toward improved diagnostics and treatment and, perhaps more critically, would support local public health agencies in obtaining resources needed to educate communities, report cases, and prevent new infections. Last, from a global health perspective, without recognizing stable transmission within its borders, the United States will be unable to reach its Sustainable Development Goals outlined in the WHO initiative Ending the Neglect to Attain Sustainable Development Goals: A Road Map for Neglected Tropical Disease 2021–2030; specifically, the third foundational pillar that centers on changing “operating models and culture to facilitate country ownership” would be unattainable (https://www.paho.org/en/topics/chagas-disease).
T. cruzi and the ecologic conditions that sustain its transmission cycles are naturally occurring throughout the southern half of the United States. Infection has been consistently demonstrated in wildlife reservoirs, companion animals, zoo and exotic mammals, and humans. At least 4 triatomine species are frequently encountered in homes and found to be harboring T. cruzi parasites. Canine Chagas disease is a concern in many working and companion dog populations in the southern United States but is likely underrecognized in many areas. The exposure of nonhuman primates to T. cruzi–infected triatomines poses a challenge to medical research. Moreover, the lack of reporting requirements for human Chagas disease adds complexity to the documentation of autochthonous cases. The number of documented autochthonous cases is higher in Texas than in other states, and cases are consistently documented each year.
This body of evidence justifies recognizing that Chagas disease is endemic to the United States, and not just from a veterinary perspective. Updating Chagas disease endemicity status as hypoendemic is a crucial step toward a more effective management model, one that addresses the unique challenges and complexities of this country regarding vectorborne diseases. Such a shift will help reform curriculum in professional schools to enable the next generation of practitioners to be competent in recognizing the low but present risk for locally acquired T. cruzi infections and better serve those who acquire the parasite elsewhere and require diagnosis in the United States. There is an opportunity to learn from the public health experiences in Mexico and other regions of Latin America that have long faced a high burden of human Chagas disease; those experiences call for increased understanding and disease management that integrates biomedical, sociocultural and policy perspectives (47). Managing endemic Chagas disease in the United States will require overcoming systemic, structural, clinical, and psychosocial healthcare barriers (48). Case investigations would ideally have standardized data collection and case classification, to include travel history, triatomine encounters, and proximity to suitable triatomine habitat, all of which are useful in assessing local transmission risk (49). In the meantime, solutions for Chagas disease can be advanced through the study of the unfortunate abundance of naturally infected animals across the southern United States (50). To achieve the Sustainable Development Goals for the 2030 Neglected Tropical Disease roadmap, recognizing Chagas disease endemicity in the United States as a regional issue will be imperative to begin implementing local, state, and national strategic plans to tackle this neglected disease that, as has been demonstrated, has never been exclusively tropical.
Dr. Beatty is an associate professor of medicine at the University of Florida College of Medicine, Department of Medicine, Division of Infectious Diseases and Global Medicine. His primary research interests are triatomine biology, Trypanosoma cruzi pathogenesis, and clinical Chagas disease.
Acknowledgment
We thank all scientists, clinicians, community health workers, and colleagues who have studied and offered data and perspective about Chagas disease in the United States. We thank Amanda Brock-Morales for assisting with the creation of Figure 1.
References
- Miranda-Arboleda AF, Zaidel EJ, Marcus R, Pinazo MJ, Echeverría LE, Saldarriaga C, et al.; Neglected Tropical Diseases and other Infectious Diseases affecting the Heart (NET-Heart) project. Roadblocks in Chagas disease care in endemic and nonendemic countries: Argentina, Colombia, Spain, and the United States. The NET-Heart project. PLoS Negl Trop Dis. 2021;15:
e0009954 . DOIPubMedGoogle Scholar - Gascon J, Bern C, Pinazo MJ. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 2010;115:22–7. DOIPubMedGoogle Scholar
- Tan MM, Matthews KRW. Misconceptions and limited awareness of Chagas disease in Texas among surveyed Houston physicians. 2018 [cited 2024 Oct 15]. https://www.bakerinstitute.org/sites/default/files/2018-12/import/chb-pub-chagas-121818.pdf
- Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis. 2010;16:871–2. DOIPubMedGoogle Scholar
- Reeves WK, Miller MM. A new state record for Triatoma sanguisuga (Leconte) (Hemiptera: Reduviidae) from Wyoming, U.S.A. Comp Parasitol. 2020;87:118–20. DOIGoogle Scholar
- Busselman RE, Hamer SA. Chagas disease ecology in the United States: recent advances in understanding Trypanosoma cruzi transmission among triatomines, wildlife, and domestic animals and a quantitative synthesis of vector-host interactions. Annu Rev Anim Biosci. 2022;10:325–48. DOIPubMedGoogle Scholar
- Beatty NL, White ZS, Bhosale CR, Wilson K, Cannella AP, Stenn T, et al. Anaphylactic reactions due to Triatoma protracta (Hemiptera, Reduviidae, Triatominae) and invasion into a home in northern California, USA. Insects. 2021;12:1018. DOIGoogle Scholar
- Beatty NL, Klotz SA. Autochthonous Chagas disease in the United States: how are people getting infected? Am J Trop Med Hyg. 2020;103:967–9. DOIPubMedGoogle Scholar
- Bern C, Messenger LA, Whitman JD, Maguire JH. Chagas disease in the United States: a public health approach. Clin Microbiol Rev. 2019;33:e00023–19. DOIGoogle Scholar
- Curtis-Robles R, Wozniak EJ, Auckland LD, Hamer GL, Hamer SA. Combining public health education and disease ecology research: using citizen science to assess Chagas disease entomological risk in Texas. PLoS Negl Trop Dis. 2015;9:
e0004235 . DOIPubMedGoogle Scholar - Beard CB, Young DG, Butler JF, Evans DA. First isolation of Trypanosoma cruzi from a wild-caught Triatoma sanguisuga (LeConte) (Hemiptera: Triatominae) in Florida, U.S.A. J Parasitol. 1988;74:343–4. DOIPubMedGoogle Scholar
- Curtis-Robles R, Hamer SA, Lane S, Levy MZ, Hamer GL. Bionomics and spatial distribution of Triatomine vectors of Trypanosoma cruzi in Texas and other southern states, USA. Am J Trop Med Hyg. 2018;98:113–21. DOIPubMedGoogle Scholar
- Dumonteil E, Tu W, Jiménez FA, Herrera C. Ecological interactions of Triatoma sanguisuga (Hemiptera: Reduviidae) and risk for human infection with Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae) in Illinois and Louisiana. J Med Entomol. 2024;61:1282–9. DOIGoogle Scholar
- Klotz SA, Smith SL, Schmidt JO. Kissing bug intrusions into homes in the Southwest United States. Insects. 2021;12:654. DOIPubMedGoogle Scholar
- Mehringer PJ Jr, Wood SF. A resampling of wood rat houses and human habitations in Griffith Park, Los Angeles, for Triatoma protracta and Trypanosoma cruzi. Bull South Calif Acad Sci. 2022;57:39–46.
- Zeledon R, Beard CB, Pinto Dias JC, Leiby DA, Dorn PL, Rodrigues Coura J. An appraisal of the status of Chagas disease in the United States. Amsterdam: Elsevier; 2012.
- Hodo CL, Hamer SA. Toward an ecological framework for assessing reservoirs of vector-borne pathogens: wildlife reservoirs of Trypanosoma cruzi across the southern United States. ILAR J. 2017;58:379–92. DOIPubMedGoogle Scholar
- Torhorst CW, White ZS, Bhosale CR, Beatty NL, Wisely SM. Identification of the parasite, Trypanosoma cruzi, in multiple tissues of epidemiological significance in the Virginia opossum (Didelphis virginiana): Implications for environmental and vertical transmission routes. PLoS Negl Trop Dis. 2022;16:
e0010974 . DOIPubMedGoogle Scholar - Majeau A, Cloherty E, Anderson AN, Straif-Bourgeois SC, Dumonteil E, Herrera C. Genetic diversity of Trypanosoma cruzi infecting raccoons (Procyon lotor) in 2 metropolitan areas of southern Louisiana: implications for parasite transmission networks. Parasitology. 2023;150:374–81. DOIPubMedGoogle Scholar
- Meyers AC, Purnell JC, Ellis MM, Auckland LD, Meinders M, Hamer SA. Nationwide exposure of U.S. working dogs to the Chagas disease parasite, Trypanosoma cruzi. Am J Trop Med Hyg. 2020;102:1078–85. DOIPubMedGoogle Scholar
- Busselman RE, Meyers AC, Zecca IB, Auckland LD, Castro AH, Dowd RE, et al. High incidence of Trypanosoma cruzi infections in dogs directly detected through longitudinal tracking at 10 multi-dog kennels, Texas, USA. PLoS Negl Trop Dis. 2021;15:
e0009935 . DOIGoogle Scholar - Curtis-Robles R, Snowden KF, Dominguez B, Dinges L, Rodgers S, Mays G, et al. Epidemiology and molecular typing of Trypanosoma cruzi in naturally-infected hound dogs and associated triatomine vectors in Texas, USA. PLoS Negl Trop Dis. 2017;11:
e0005298 . DOIGoogle Scholar - Gürtler RE, Cardinal MV. Dogs and their role in the eco-epidemiology of Chagas disease. In: Strube C, Mehlhorn H, editors. Dog parasites endangering human health. Cham: Springer International Publishing; 2021. p. 73–106.
- Curtis-Robles R, Zecca IB, Roman-Cruz V, Carbajal ES, Auckland LD, Flores I, et al. Trypanosoma cruzi (agent of Chagas disease) in sympatric human and dog populations in “colonias” of the Lower Rio Grande Valley of Texas. Am J Trop Med Hyg. 2017;96:805–14. DOIGoogle Scholar
- Garcia MN, O’Day S, Fisher-Hoch S, Gorchakov R, Patino R, Feria Arroyo TP, et al. One health interactions of Chagas disease vectors, canid hosts, and human residents along the Texas-Mexico border. PLoS Negl Trop Dis. 2016;10:
e0005074 . DOIGoogle Scholar - Beard CB, Pye G, Steurer FJ, Rodriguez R, Campman R, Peterson AT, et al. Chagas disease in a domestic transmission cycle, southern Texas, USA. Emerg Infect Dis. 2003;9:103–5. DOIGoogle Scholar
- Burkholder JE, Allison TC, Kelly VP. Trypanosoma cruzi (Chagas) (Protozoa: Kinetoplastida) in invertebrate, reservoir, and human hosts of the lower Rio Grande valley of Texas. J Parasitol. 1980;66:305–11. DOIPubMedGoogle Scholar
- Dorn PL, Daigle ME, Combe CL, Tate AH, Stevens L, Phillippi-Falkenstein KM. Low prevalence of Chagas parasite infection in a nonhuman primate colony in Louisiana. J Am Assoc Lab Anim Sci. 2012;51:443–7.PubMedGoogle Scholar
- Kiehl WM, Hodo CL, Hamer GL, Hamer SA, Wilkerson GK. Exclusion of horizontal and vertical transmission as major sources of Trypanosoma cruzi infections in a breeding colony of rhesus macaques (Macaca Mulatta). Comp Med. 2023;73:229–41. DOIGoogle Scholar
- Lynn MK, Bossak BH, Sandifer PA, Watson A, Nolan MS. Contemporary autochthonous human Chagas disease in the USA. Acta Trop. 2020;205:
105361 . DOIGoogle Scholar - Council of State and Territorial Epidemiologists. Standardized surveillance case definition for acute, congenital, and chronic trypanosoma cruzi infection or Chagas disease. 2024 [cited May 2025]. https://cdn.ymaws.com/www.cste.org/resource/resmgr/position_statements_files_2023/24-ID-04_Chagas_disease.pdf
- Woody NC, Woody HB. American trypanosomiasis (Chagas’ disease); first indigenous case in the United States. J Am Med Assoc. 1955;159:676–7. DOIGoogle Scholar
- Dittmar KJA, Araujo A, Reinhard KJ, Ferreira LF, Whiting A. Molecular diagnosis of prehistoric Trypanosoma cruzi in the Texas-Coahuila border region. Presented at: 13th Annual Meeting of the Paleopathology Association; Tempe, Arizona, USA; April 23–26, 2003.
- Klotz SA, Dorn PL, Klotz JH, Pinnas JL, Weirauch C, Kurtz JR, et al. Feeding behavior of triatomines from the southwestern United States: an update on potential risk for transmission of Chagas disease. Acta Trop. 2009;111:114–8. DOIGoogle Scholar
- Killets KC, Wormington J, Zecca I, Chaves LF, Hamer GL, Hamer SA. Comparative feeding and defecation behaviors of Trypanosoma cruzi-infected and uninfected triatomines (Hemiptera: Reduviidae) from the Americas. Insects. 2025;16:188. DOIGoogle Scholar
- Hudson FP, Homer N, Epstein A, Mondy K. Acute Chagas disease manifesting as orbital cellulitis, Texas, USA. Emerg Infect Dis. 2021;27:2937–9. DOIGoogle Scholar
- Turabelidze G, Vasudevan A, Rojas-Moreno C, Montgomery SP, Baker M, Pratt D, et al. Autochthonous Chagas disease—Missouri, 2018. MMWR Morb Mortal Wkly Rep. 2020;69:193–5. DOIPubMedGoogle Scholar
- Franco-Paredes C, Villamil-Gómez WE, Schultz J, Henao-Martínez AF, Parra-Henao G, Rassi A Jr, et al. A deadly feast: elucidating the burden of orally acquired acute Chagas disease in Latin America—public health and travel medicine importance. Travel Med Infect Dis. 2020;36:
101565 . DOIGoogle Scholar - Barr SC. Canine Chagas’ disease (American trypanosomiasis) in North America.[ [v–vi.]. Vet Clin North Am Small Anim Pract. 2009;39:1055–64. DOIGoogle Scholar
- Reifler K, Campbell JI, Barnett ED, Bourque DL, Hamer DH, Samra H, et al. Diagnosing Chagas in pregnancy and childhood: what’s old and new. Clin Lab Med. 2025;45:73–86. DOIGoogle Scholar
- Dicker RC, Coronado F, Koo D, Parrish RG. Principles of epidemiology in public health practice; an introduction to applied epidemiology and biostatistics. 3rd ed. 2006 [cited 2024 Oct 15]. https://stacks.cdc.gov/view/cdc/6914
- Phalkey R, Bradley N, Dobney A, Murray V, O’Hagan J, Ahmad M, et al. Human physiology, hazards and health risks. In: Battersby S, editor. Clay’s handbook of environmental health. New York: Routledge; 2022. p. 190–207.
- World Health Organization (WHO). WHO malaria terminology, 2021 update [cited 2024 Oct 15]. https://www.who.int/publications/i/item/9789240038400
- Gavic EA, Achen SE, Fox PR, Benjamin EJ, Goodwin J, Gunasekaran T, et al. Trypanosoma cruzi infection diagnosed in dogs in nonendemic areas and results from a survey suggest a need for increased Chagas disease awareness in North America. J Am Vet Med Assoc. 2023;261:705–12. DOIPubMedGoogle Scholar
- Stigler Granados P, Pacheco GJ, Núñez Patlán E, Betancourt J, Fulton L. Assessing the effectiveness of Chagas disease education for healthcare providers in the United States. BMC Infect Dis. 2020;20:743. DOIPubMedGoogle Scholar
- Levin LG, Kreimer PR, Jensen P. Chagas Disease across contexts: scientific knowledge in a globalized world. Med Anthropol. 2021;40:572–89. DOIGoogle Scholar
- Aké-Chan M, Sanmartino M, Castillo-Burguete MT, González-Martínez A, Ibarra-Cerdeña CN. (In)coherence between Chagas disease policy and the experiences of those affected in Mexico: the need for a transdisciplinary approach. PLoS Negl Trop Dis. 2025;19:
e0013052 . DOIGoogle Scholar - Forsyth C, Meymandi S, Moss I, Cone J, Cohen R, Batista C. Proposed multidimensional framework for understanding Chagas disease healthcare barriers in the United States. PLoS Negl Trop Dis. 2019;13:
e0007447 . DOIPubMedGoogle Scholar - Lund AJ, Metzger ME, Kramer VL, Kjemtrup AM. Low risk for locally acquired Chagas disease in California: A review of human cases and triatomine submissions, 2013-2023. PLoS Negl Trop Dis. 2025;19:
e0013036 . DOIPubMedGoogle Scholar - Tarleton RLSA, Lococo B, Alvarez Gianni MG, Laucella S, Hodo CL, Wilkerson GK, et al. The unfortunate abundance of Trypanosoma cruzi in naturally infected dogs and monkeys provides unique opportunities to advance solutions for Chagas disease. Zoonoses Public Health. 2024;4.
Figures
Cite This ArticleOriginal Publication Date: August 13, 2025
1All authors contributed equally to this article.
Table of Contents – Volume 31, Number 9—September 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sarah A. Hamer, Department of Veterinary Integrative Biosciences, 667 Raymond Stotzer Ave, Texas A&M University, College Station, TX 77843, USA
Top