Volume 31, Number 9—September 2025
Research
Insights into Infant Strongyloidiasis, Papua New Guinea
Abstract
The human-infecting parasite Strongyloides fuelleborni subspecies kellyi has been reported from the island of New Guinea. We analyzed fecal DNA extracts (n = 164) from 19 infants in Papua New Guinea by using Strongyloides real-time PCR and undertook metabarcoding of cox1 and 18S rRNA hypervariable regions I and IV loci. Eight infants were infected with Strongyloides spp.; 7 were infected with S. fuelleborni subsp. fuelleborni and 1 with a Strongyloides sp. previously misattributed to S. fuelleborni subsp. kellyi. Phylogenetic and haplotyping analyses indicated S. fuelleborni in Papua New Guinea belongs to the Indochina subclade of S. fuelleborni subsp. fuelleborni and is not a unique subspecies. We report molecular evidence of S. fuelleborni subsp. fuelleborni infection in humans in the Pacific. Our findings also demonstrate the potential co-existence of an undescribed human-infecting Strongyloides sp. on the island of New Guinea, indicating a need for renewed clinical and epidemiologic investigations into infant strongyloidiasis.
New Guinea, consisting of Indonesian New Guinea in the west and Papua New Guinea in the east, is the world’s largest tropical island and a biodiversity hotspot. The island is home to the widespread and well-understood human pathogen S. stercoralis but also to a unique and enigmatic agent of human strongyloidiasis, the nematode Strongyloides fuelleborni subspecies kellyi (1–3). This S. fuelleborni–like intestinal nematode of humans was first reported in western Papua New Guinea in 1971 (4) and later found in Indonesian New Guinea (5).
There was taxonomic confusion over the identity of the New Guinea Strongyloides and its relationship to S. fuelleborni from Africa. Unlike S. stercoralis, which is passed as rhabditiform larvae, S. fuelleborni subsp. kellyi is passed as eggs, resembling those of larvated hookworms, in feces (1,6). Viney et al. observed the adult nematodes were morphologically distinguishable by subtle differences in the peri-vulval cuticle of parasitic female specimens and the phasmidial pore position of free-living male specimens (3). A separate isoenzyme electrophoresis analysis revealed that the 2 worm isolates clustered closely, separate from other mammal-infecting Strongyloides spp. (7). On the basis of those findings, subspeciation of S. fuelleborni into 2 subspecies was proposed (3): S. fuelleborni subsp. fuelleborni, and S. fuelleborni subsp. kellyi for the New Guinea nematode. Of note, strains of S. fuelleborni from Asia were not included in that analyses (3,7).
The epidemiology of S. fuelleborni subsp. kellyi nematodes is similarly enigmatic. Infection in children has been observed within 3 weeks after birth (6). One study found the incidence of infection rose rapidly in the first 12 months of life and then stabilized until 5 years of age, at which age-related incidence began to drop (8). How infection occurs in children so young is unclear. Transmammary transmission has been suggested (6), although a screening of breastmilk from 40 mothers in an endemic village during the 1970s revealed no larvae (9). Nonhuman primates, the animal reservoir of S. fuelleborni subsp. fuelleborni, are not native to New Guinea, and attempts to find an animal reservoir for S. fuelleborni subsp. kellyi by screening domestic animals, including pigs, chickens, and dogs from villages where infections occurred at high prevalence in humans, were unsuccessful (4,7).
Heavy S. fuelleborni subsp. kellyi infection has been implicated as the cause of swollen belly syndrome (SBS), a rapidly fatal disease in infants around 2 months of age (6). SBS is a protein-losing enteropathy characterized by eosinophilia, distended abdomen, diarrhea, and respiratory distress (10). The etiology of SBS remains poorly understood. SBS was observed during 1974–1983, predominantly in 2 remote villages in mountainous regions of Papua New Guinea, Kanabea (Gulf Province, ≈1270 m above sea level) and Wanuma (Madang Province, ≈750 m above sea level). Only occasional, sporadic cases were reported elsewhere (6). In Kanabea, infection with an egg-producing Strongyloides sp. was found in 100% of infants 3–8 weeks after birth, with a mean average egg burden of 94,000 eggs/mL of feces (9). SBS caused 8% of infant deaths in this village until specific anthelmintic therapy was introduced (6). This possible correlation of Strongyloides sp. infection to SBS is despite reports of high burdens and prevalence of Strongyloides eggs, presumed to be S. fuelleborni subsp. kellyi, in infants across several other regions of Papua New Guinea, where infections were associated with nutritional deficiencies but not SBS (11,12). An unknown factor was proposed to be involved in the development of SBS (10).
We found only 1 S. fuelleborni subsp. kellyi DNA sequence recorded to date (13), a 330-bp segment of 18S (small subunit) rRNA that covers the hypervariable region (HVR) I (14). Phylogenetic analysis on the basis of this region placed S. fuelleborni subsp. kellyi in a clade with S. cebus, S. papillosus, and S. venezuelensis but distant from S. fuelleborni subsp. fuelleborni. That work prompted calls to elevate S. fuelleborni subsp. kellyi to the species rank (2). However, a criticism of that finding is that 18S rRNA HVR-I is a poor marker for inferring taxonomic positions of Strongyloides spp (15,16). That gene region did not enable the separation of Strongyloides spp. with spiraled and straight ovary morphotypes, whereas nearly full-length 18S rRNA and 28S rRNA sequences did (13,17). Furthermore, 1 study (13) found S. fuelleborni subsp. fuelleborni to be phylogenetically closer to S. stercoralis than to S. papillosus, contradicting evidence suggested by mitochondrial genome data (18). Molecular taxonomy of Strongyloides spp. determined by using markers such as mitochondrial cytochrome oxidase subunit 1 (cox1) and 18S rRNA HVR-IV showed considerable consistency with whole-genome and mitochondrial genome analyses (18,19). The true phylogeny and taxonomic identity of S. fuelleborni subsp. kellyi could be elucidated by using those informative markers.
Despite its public health significance, S. fuelleborni subsp. kellyi remains a neglected and underexplored human helminth. This enigmatic causative agent of strongyloidiasis in New Guinea warrants focused research to clarify its identity and epidemiology. We analyzed fecal DNA extracts from 19 infants in Papua New Guinea to explore the identity of S. fuelleborni subsp. kellyi infection.
Sampling
We obtained samples from a 1-year longitudinal study investigating gut health in infants in Eastern Highlands Province, Papua New Guinea, during 2018–2019 (Figure 1). Ethics approval was obtained from the Papua New Guinea Institute of Medical Research Institutional Review Board (approval no. 1614) and the Papua New Guinea Medical Research Advisory Committee (approval no. 17.17). Infants were enrolled into the study within the first 5 weeks after birth. Initially, parents gave consent for participation in the study during antenatal clinic visits; after birth, informed consent was obtained from >1 parent or legal guardian. Fecal samples were collected from participants monthly for 12 months.
For our study, we investigated the presence of Strongyloides spp. in fecal samples from 19 total infants, 12 boys and 7 girls. For samples used in our study, infants were 0–101 days (average 30 days) of age when their first fecal sample was collected. Clinical data on the participants was limited, but no marked gastrointestinal pathology was noted during the study.
Fresh fecal samples were transported to the Papua New Guinea Institute of Medical Research (Goroka, Papua New Guinea), where they were stored at −70°C. Samples were subsequently sent to Queensland Institute of Medical Research-Berghofer Medical Research Institute (Herston, Queensland, Australia) and James Cook University (Townsville, Queensland, Australia) for laboratory analysis. We extracted DNA from each sample by using a QIAamp DNA mini kit (QIAGEN, https://www.qiagen.com). Before extraction, we mixed fecal samples (≈200 mg) with 500 µL of rapid 1-step extraction buffer, 15 µL of proteinase K, and 500 µL of 0.5-mm silica/zirconia beads (Daintree Scientific, http://www.daintreescientific.com.au) in 2-mL screw-cap tubes (Starstedt, https://www.sarstedt.com). We homogenized the tubes at 6,500 rpm for 40 seconds by using a Precellys homogenizer (Bertin Technologies, https://www.bertin-technologies.com). After homogenization, we incubated the samples at 95°C for 5 minutes and then centrifuged at 4,000 relative centrifugal force for 3 minutes. We transferred the supernatant to a new 1.5-mL tube and then followed the QIAGEN protocol for DNA extraction.
We assessed the extracted fecal DNA for quality and quantity by using a NanoDrop 2000 (Thermo Fisher Scientific, https://www.thermofisher.com). We used DNA samples with a concentration >10 ng/µL, and a 260/280 ratio between 1.6 and 2.10 as quantitative PCR templates. We reextracted 5 samples that did not meet those requirements; all subsequently reached the required DNA concentration. We then diluted the DNA 1:5 with Milli Q water (Thermo Fisher Scientific) and subjected it to 2 Strongyloides qPCRs (20,21). We confirmed positive qPCR results by triplicate testing and samples were considered positive when the cycle threshold (Ct) was <35. To tentatively infer the identity of the qPCR product, we conducted Sanger sequencing of positive amplicons from the 2 qPCRs, targeting a 471-bp region of 18S rRNA (20) and a 138-bp repeat sequence (21) by using the BigDye Direct Cycle Sequencing kit (Thermo Fisher Scientific).
We also performed a metabarcoding assay targeting 18S rRNA HVR-IV (≈252-bp) by using the Illumina MiSeq Reagent Nano Kit v2 (Illumina, https://www.illumina.com) (16). We then subjected positive samples to additional metabarcoding of the 18S rRNA HVR-I (≈432-bp) and partial cox1 (217-bp) genotyping targets (16) by using the same Illumina MiSeq platform. We conducted sequencing with 500 cycles (paired-end, 250-bp) for 18S rRNA products and 300 cycles (paired-end, 150-bp) for the cox1 products to ensure adequate sequencing coverage.
Statistical and Bioinformatic Analysis
We imported demographic and qPCR data into Excel (Microsoft, https://www.microsoft.com) for statistical analysis. We had the raw Illumina sequencing data analyzed by 3 blinded researchers at James Cook University and the Institute of Vertebrate Biology (IVB) through 2 different computational pipelines: a custom Geneious Prime (https://www.geneious.com) workflow as previously published (16) at both James Cook University and IVB, and a combination of Skewer (22) and DADA2 (23) packages implemented in R software version 4.2.2 (The R Project for Statistical Computing, https://www.r-project.org) at IVB. Both pipelines incorporated read quality control, contig assembly, and haplotype assignment. We conducted maximum-likelihood phylogenetic analysis in MEGA 11 (https://www.megasoftware.net) and Bayesian inference phylogenetic analysis in MrBayes (https://nbisweden.github.io/MrBayes) on MUSCLE-aligned (https://github.com/rcedgar/muscle) cox1 sequences by using the general time-reversible model for nucleotide substitutions. We then used Cohen’s κ to assess the diagnostic agreement between different molecular methods for the detection of Strongyloides spp.
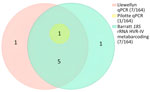
A total of 164 fecal samples were collected from 19 infants during 5–13 occasions over the study period (Figure 1, 2). Eight (42%) of the 19 infants, comprising 6 boys and 2 girls, were found to be infected with a Strongyloides spp. Infections were detected at an average age of 212 days (range 94–334 days) (Figure 2). Of the 8 positive cases, fecal metabarcoding at cox1 and 18S rRNA HVR-I and HVR-IV loci identified 6 infections as S. fuelleborni subsp. fuelleborni and 1 as a Strongyloides sp. previously attributed to S. fuelleborni subsp. kellyi (13) (Table). The remaining positive sample could only be amplified at the qPCR 18S rRNA target. That 471-bp sequence was 99.2% identical to a published S. fuelleborni subsp. fuelleborni sequence (GenBank accession no. AB272235) by BLAST analysis (https://blast.ncbi.nlm.nih.gov/).
The previously published genus-specific qPCR method (20) performed comparably with a previously published 18S rRNA HVR-IV metabarcoding method (16) in detecting Strongyloides infections, yielding a Cohen’s κ coefficient of 0.85. The second qPCR method (21) did not detect S. fuelleborni subsp. fuelleborni but did detect the Strongyloides spp. previously considered S. fuelleborni subsp. kellyi (Figure 3).
We obtained sequences of 18S rRNA HVR-I (432-bp) and HVR-IV (≈252-bp) from 7 samples (Table). Six positive samples harbored HVR-IV haplotype S and HVR-I haplotype XIV, both genotypes previously identified in all S. fuelleborni subsp. fuelleborni isolates from Asia. The remaining positive sample was infected with 3 HVR-I haplotypes (deposited into GenBank under accession nos. PV489780–2): two with 275-bp sequences identical to the only published sequence of S. fuelleborni subsp. kellyi (GenBank accession no. AJ417029) (13) and the third differing by 1 single-nucleotide polymorphism (SNP) (T-C at position 28). HVR-I sequences of that Strongyloides sp. (432-bp) differed from those of S. ransomi (GenBank accession nos. LC324901, OP288111) by 2 SNPs and from S. venezuelensis (GenBank accession no. AB923887) by 1 SNP (Figure 4). At the HVR-IV locus, the 248-bp sequence was 100% identical to sequences of S. ransomi (GenBank accession nos. OP288111, KU724127) and S. venezuelensis (GenBank accession no. AB923887).
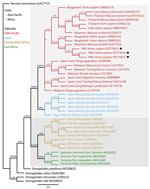
cox1 sequences were available for 6 samples, all assigned to S. fuelleborni subsp. fuelleborni (Table). The seventh sample, containing the genetically distinct Strongyloides sp., did not amplify. We identified 3 separate S. fuelleborni subsp. fuelleborni haplotypes (deposited into GenBank accession nos. PQ774615–7). Maximum-likelihood and Bayesian inference phylogenetic analyses on the cox1 locus placed S. fuelleborni subsp. fuelleborni from Papua New Guinea in a clade with S. fuelleborni subsp. fuelleborni from Myanmar rhesus macaques (GenBank accession no. OL672153). Those sequences also clustered closely with S. fuelleborni subsp. fuelleborni from Bangladesh (GenBank accession nos. OR805176 and OR805181) (Figure 5).
We provide molecular evidence of human infections with S. fuelleborni subsp. fuelleborni outside of Asia and Africa. The potential existence of an undescribed human-infecting Strongyloides sp., previously misattributed to S. fuelleborni subsp. kellyi (13), is also suggested. On the basis of those findings, we hypothesize that >2 genetically distinct, non–S. stercoralis Strongyloides nematodes infect humans in Papua New Guinea and that S. fuelleborni subsp. kellyi, as previously described (3), is not a unique subspecies but is likely synonymous with the Asia-Pacific clade of S. fuelleborni subsp. fuelleborni.
Our phylogenetic findings corroborate a previously published study (3) that described very subtle morphologic distinctions between S. fuelleborni adult isolates from Africa and Papua New Guinea. Both strains differed markedly in morphology from S. ransomi in Papua New Guinea pigs. Because those analyses did not include isolates of S. fuelleborni subsp. fuelleborni from Asia, and no morphologic studies of this nematode from Asia exist, it is possible that study described the first representatives of what is now recognized as the clade of S. fuelleborni subsp. fuelleborni from Asia (3). Further comparative morphologic and genomic analyses of adult isolates from Africa, Asia, and the Pacific are required to test that hypothesis. If confirmed, the finding would warrant a revision of current subspecies epithets to reflect the divergence between Africa and Asia-Pacific S. fuelleborni.
In Africa and Asia, S. fuelleborni subsp. fuelleborni is a common infection of nonhuman primates and is considered a zoonosis originating from those animals (24,25). Multiple studies suggest human-to-human transmission of S. fuelleborni subsp. fuelleborni occurs in some regions of Africa (25,26). This suggestion is supported by genetic analysis (27) identifying a human-specific subpopulation among S. fuelleborni subsp. fuelleborni isolates from Africa. Because of the absence of a nonhuman primate reservoir on the island of New Guinea, it is likely that S. fuelleborni subsp. fuelleborni has adapted to exclusive human-to-human transmission after being introduced to Papua New Guinea through human migration (28).
The distinct Strongyloides genospecies identified in 1 infant from this study corresponds to the genospecies previously misattributed to S. fuelleborni subsp. kellyi (13). This species was indistinguishable from S. ransomi and S. venezuelensis at the 18S rRNA HVR-IV locus and exhibited only 1–2 SNPs at the HVR-I locus, suggesting a recent common ancestry among them. An earlier isoenzyme electrophoretic analysis (7) found that 4 of 26 Strongyloides isolates recovered from persons in Papua New Guinea clustered closely with S. ransomi from local pigs. Although there was speculation those samples might have originated from pigs, it is plausible they represented the same distinct species identified in this and a previous study (13). However, caution is warranted when interpreting taxonomic placements based solely on single-locus 18S rRNA data from fecal DNA, without accompanying morphologic data, because those inferences might be artifactual. Detailed morphologic analysis of adult isolates, combined with whole-genome or mitochondrial genome sequencing, is needed to resolve the taxonomic status of this nematode.
Historical reports of S. fuelleborni subsp. kellyi predated the molecular era and relied solely on microscopic identification of Strongyloides eggs (4,6,8,9,12,28). Those data require reassessment because they might conflate infections with 2 co-endemic human-infecting Strongyloides spp. nematodes. Surveys conducted during 1976–1997 in Papua New Guinea reported S. fuelleborni subsp. kellyi prevalence of 20%–93% in children, with rates reaching 60% within the first year of life (6,8,9,12,28). We similarly found a high incidence of S. fuelleborni infection (7/19), but also 1 infection (1/19) with an undescribed Strongyloides sp. in Papua New Guinea infants. Future surveillance for strongyloidiasis in New Guinea should use species-specific molecular tools to differentiate those 2 agents.
Our findings provide explanation for much of the unknown epidemiology of infant strongyloidiasis in Papua New Guinea. Patent S. fuelleborni infections in infants as young as 18 days of age have been found in Papua New Guinea (6). Sampling of breastmilk from mothers in Papua New Guinea (29) did not identify any Strongyloides larvae; however, difficulties in obtaining fecal samples from those mothers left their infection status uncertain. In the same village, the prevalence of Strongyloides spp. in adult feces was only 14% (12). In a survey of 25 lactating mothers of infants with confirmed S. fuelleborni subsp. fuelleborni infection in the Democratic Republic of the Congo, Strongyloides spp. filariform larvae were found in the breast milk of 1 mother (30). That finding suggests transmammary transmission could be responsible for the high rate of infection in infants as young as 50–74 days of age in that region (30). Because our genetic analysis indicates the worms we identified belong to clades of the same species, it is plausible that transmammary transmission to Papua New Guinea infants might occur. That speculation warrants further investigation, and the use of molecular genotyping tools might be necessary to track transmission patterns.
The attribution of S. fuelleborni subsp. kellyi as the causative agent of infantile SBS requires further validation. Despite S. fuelleborni infection being widespread in children in some parts of Africa, SBS has not been reported from that continent (25). Neither has SBS associated with S. fuelleborni infection been documented in Asia. The detection of a Strongyloides genospecies closely related to S. ransomi of pigs raises an alternative hypothesis regarding the etiology of SBS in Papua New Guinea. In newborn suckling piglets, S. ransomi infection causes a protein-losing enteropathy characterized by villus atrophy, malabsorption, diarrhea, progressive dehydration, hypoproteinaemia, anemia, anorexia, emaciation, sudden death (31,32), and reduced hepatic protein synthesis (33), a clinical picture strikingly similar to SBS in infants from Papua New Guinea (1,6). Because of the substantial genetic similarity observed between S. ransomi and this genospecies, a shared pathogenic mechanism is plausible. S. ransomi detection in only 1 of 19 infants also mirrors the rare and sporadic occurrence of SBS previously reported in this population (6,9,10). Further research is needed to explore this hypothesis and clarify the etiology and epidemiology of SBS.
In our study, not all diagnostic Strongyloides spp. qPCRs detected S. fuelleborni infection. The Llewelyn modification (20) of Verweij (34) qPCR appears generic and detected all except 1 S. fuelleborni infection. In contrast, the Pilotte S. stercoralis qPCR (21) only amplified DNA of the undescribed Strongyloides sp. and did not detect S. fuelleborni infections. That finding demonstrates that the specificity of PCR diagnostics used must be considered in future surveillance of Strongyloides spp. infections in humans and animals, because the choice of qPCR might markedly affect the findings of any survey.
In summary, we present molecular evidence of human infections with S. fuelleborni subsp. fuelleborni nematodes in Papua New Guinea. On the basis of this evidence and existing morphologic data on S. fuelleborni subsp. kellyi, we hypothesize that S. fuelleborni subsp. kellyi is not a unique subspecies but rather represents S. fuelleborni subsp. fuelleborni Asia-Pacific clade infections occurring in humans in New Guinea. We further molecularly identified an undescribed Strongyloides species in 1 infant from Papua New Guinea and raise questions about the possible role of this undescribed species in the etiology of infantile SBS. Renewed clinical, epidemiologic and taxonomic investigations into infant strongyloidiasis in this region are needed to increase clinician awareness of such infections and guide prevention and treatment efforts.
Ms. Zhao is a PhD candidate at James Cook University. Her research focuses on the molecular taxonomy and epidemiology of Strongyloides species in humans and companion animals.
Acknowledgments
We thank the participants and their families for providing stool samples. We thank the leadership and staff at Papua New Guinea Institute of Medical Research, particularly members of the Infection and Immunity Unit, for their collaboration on this project.
This work was partially funded by the National Health and Medical Research Council (investigator grant no. APP1194462). H.Z. and J.H. receive an Australian Government Research Training Program scholarship from James Cook University, Australia.
References
- Bradbury RS. Strongyloides fuelleborni kellyi in New Guinea: neglected, ignored and unexplored. Microbiol Aust. 2021;42:169–72. DOIGoogle Scholar
- Page W, Shield J, O’Donahoo F, Miller A, Judd J, Speare R. Strongyloidiasis in Oceania. In: Hotez PT, editor. Neglected tropical diseases-Oceania. Switzerland: Springer; 2016. p. 69–99.
- Viney M, Ashford R, Barnish G. A taxonomic study of Strongyloides Grassi, 1879 (nematoda) with special reference to Strongyloides fuelleborni von Linstow, 1905 in man in Papua New Guinea and the description of a new subspecies. Syst Parasitol. 1991;18:95–109. DOIGoogle Scholar
- Kelly A, Voge M. Report of a nematode found in humans at Kiunga, Western District. P N G Med J. 1973;16:59.
- Muller R, Lillywhite J, Bending JJ, Catford JC. Human cysticercosis and intestinal parasitism amongst the Ekari people of Irian Jaya. J Trop Med Hyg. 1987;90:291–6.PubMedGoogle Scholar
- Ashford RW, Barnish G, Viney ME. Strongyloides fuelleborni kellyi: infection and disease in Papua New Guinea. Parasitol Today. 1992;8:314–8. DOIPubMedGoogle Scholar
- Viney ME, Ashford RW. The use of isoenzyme electrophoresis in the taxonomy of Strongyloides. Ann Trop Med Parasitol. 1990;84:35–47. DOIPubMedGoogle Scholar
- Barnish G, Ashford RW. Strongyloides cf fuelleborni in Papua New Guinea: epidemiology in an isolated community, and results of an intervention study. Ann Trop Med Parasitol. 1989;83:499–506. DOIPubMedGoogle Scholar
- Ashford RW, Vince JD, Gratten MJ, Bana-Koiri J. Strongyloides infection in a mid-mountain Papua New Guinea community: results of an epidemiological survey. 1979. P N G Med J. 2005;48:58–65.PubMedGoogle Scholar
- Vince JD, Ashford RW, Gratten MJ, Bana-Koiri J. Strongyloides species infestation in young infants of Papua New Guinea: association with generalized oedema. 1979. P N G Med J. 2005;48:50–7.PubMedGoogle Scholar
- Barnish G, Harari M. Possible effects of Strongyloides fuelleborni-like infections on children in the Karimui area of Simbu Province. P N G Med J. 1989;32:51–4.PubMedGoogle Scholar
- King SE, Mascie-Taylor CGS. Strongyloides fuelleborni kellyi and other intestinal helminths in children from Papua New Guinea: associations with nutritional status and socioeconomic factors. P N G Med J. 2004;47:181–91.PubMedGoogle Scholar
- Dorris M, Viney ME, Blaxter ML. Molecular phylogenetic analysis of the genus Strongyloides and related nematodes. Int J Parasitol. 2002;32:1507–17. DOIPubMedGoogle Scholar
- Hasegawa H, Hayashida S, Ikeda Y, Sato H. Hyper-variable regions in 18S rDNA of Strongyloides spp. as markers for species-specific diagnosis. Parasitol Res. 2009;104:869–74. DOIPubMedGoogle Scholar
- Aupalee K, Wijit A, Singphai K, Rödelsperger C, Zhou S, Saeung A, et al. Genomic studies on Strongyloides stercoralis in northern and western Thailand. Parasit Vectors. 2020;13:250. DOIPubMedGoogle Scholar
- Barratt JLN, Lane M, Talundzic E, Richins T, Robertson G, Formenti F, et al. A global genotyping survey of Strongyloides stercoralis and Strongyloides fuelleborni using deep amplicon sequencing. PLoS Negl Trop Dis. 2019;13:
e0007609 . DOIPubMedGoogle Scholar - Hino A, Tanaka T, Takaishi M, Fujii Y, Palomares-Rius JE, Hasegawa K, et al. Karyotype and reproduction mode of the rodent parasite Strongyloides venezuelensis. Parasitology. 2014;141:1736–45. DOIPubMedGoogle Scholar
- Ko PP, Haraguchi M, Hara T, Hieu DD, Ito A, Tanaka R, et al. Population genetics study of Strongyloides fuelleborni and phylogenetic considerations on primate-infecting species of Strongyloides based on their mitochondrial genome sequences. Parasitol Int. 2023;92:
102663 . DOIPubMedGoogle Scholar - de Ree V, Nath TC, Barua P, Harbecke D, Lee D, Rödelsperger C, et al. Genomic analysis of Strongyloides stercoralis and Strongyloides fuelleborni in Bangladesh. PLoS Negl Trop Dis. 2024;18:
e0012440 . DOIPubMedGoogle Scholar - Llewellyn S, Inpankaew T, Nery SV, Gray DJ, Verweij JJ, Clements AC, et al. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Negl Trop Dis. 2016;10:
e0004380 . DOIPubMedGoogle Scholar - Pilotte N, Papaiakovou M, Grant JR, Bierwert LA, Llewellyn S, McCarthy JS, et al. Improved PCR-based detection of soil transmitted helminth infections using a next-generation sequencing approach to assay design. PLoS Negl Trop Dis. 2016;10:
e0004578 . DOIPubMedGoogle Scholar - Jiang H, Lei R, Ding S-W, Zhu S. Skewer: a fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinformatics. 2014;15:182. DOIPubMedGoogle Scholar
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. DOIPubMedGoogle Scholar
- Janwan P, Rodpai R, Intapan PM, Sanpool O, Tourtip S, Maleewong W, et al. Possible transmission of Strongyloides fuelleborni between working Southern pig-tailed macaques (Macaca nemestrina) and their owners in Southern Thailand: Molecular identification and diversity. Infect Genet Evol. 2020;85:
104516 . DOIPubMedGoogle Scholar - Pampiglione S, Ricciardi M. Geographic distribution of Strongyloides fulleborni in humans in tropical Africa. Parasitologia. 1972;14:329–38.
- Hira PR, Patel BG. Human strongyloidiasis due to the primate species Strongyloides fülleborni. Trop Geogr Med. 1980;32:23–9.PubMedGoogle Scholar
- Barratt JLN, Sapp SGH. Machine learning-based analyses support the existence of species complexes for Strongyloides fuelleborni and Strongyloides stercoralis. Parasitology. 2020;147:1184–95. DOIPubMedGoogle Scholar
- Kelly A, Little MD, Voge M. Strongyloides fulleborni-like infections in man in Papua New Guinea. Am J Trop Med Hyg. 1976;25:694–9. DOIPubMedGoogle Scholar
- Barnish G, Ashford RW. Strongyloides cf. fuelleborni and hookworm in Papua New Guinea: patterns of infection within the community. Trans R Soc Trop Med Hyg. 1989;83:684–8. DOIPubMedGoogle Scholar
- Brown RC, Girardeau HF. Transmammary passage of Strongyloides sp. larvae in the human host. Am J Trop Med Hyg. 1977;26:215–9. DOIPubMedGoogle Scholar
- Constable P, Hinchcliff K, Done S, Grünberg W. Diseases of the alimentary tract: nonruminant. Veterinary medicine. 11th ed. St. Louis: Elsevier; 2017.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie M, editor. Jubb, Kennedy & Palmer’s pathology of domestic animals. 6th ed. Philadelphia: W.B. Saunders; 2016. p. 1–257.e2.
- Dey-Hazra A, Sallmann HP, Enigk K, Harisch G. Protein synthesis changes in the liver of piglets infected with Strongyloides ransomi. Vet Parasitol. 1979;5:339–51. DOIGoogle Scholar
- Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009;103:342–6. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: August 19, 2025
Table of Contents – Volume 31, Number 9—September 2025
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Richard S. Bradbury, School of Public Health and Tropical Medicine, College of Medicine and Dentistry, Bldg 41, University Drive, James Cook University, Townsville, QLD 4811, Australia
Top