Volume 31, Number 9—September 2025
Dispatch
Emergence of Autochthonous Leishmania (Mundinia) martiniquensis Infections in Horses, Czech Republic and Austria, 2019–2023
Abstract
We report 4 cases of equine cutaneous leishmaniasis caused by Leishmania martiniquensis in Czech Republic and Austria, outside the known endemic range of leishmaniases. The parasite should be considered as a potential cause of cutaneous lesions in horses; the risk for zoonotic transmission to immunocompromised humans is anticipated throughout central Europe.
Leishmaniasis is a relatively rare equine disease caused by several Leishmania spp. protozoan parasites. In Mediterranean Europe, clinical leishmaniasis in animals (mainly domestic carnivores) and humans is primarily caused by L. infantum. In areas endemic for L. infantum, sporadic cases of leishmaniosis in horses have also been reported, typically manifesting as ulcerating cutaneous nodules (1). During 2002–2010, cases of leishmaniosis were reported in horses (2) and cattle (3) in areas north of the Alps, which are considered nonendemic because of the low abundance of L. infantum vectors. Those sporadic cases were initially attributed to L. siamensis but were later reclassified as L. martiniquensis (4).
L. martiniquensis, a member of the subgenus Mundinia, is a zoonotic species originally described from a human visceral case in the Caribbean (5). L. martiniquensis parasites have wide distribution, spanning >3 continents, overlapping with other Leishmania species in many areas, including Europe (6). However, the full host range and epidemiology remain unclear. The distribution of cases outside the range of Phlebotomus/Lutzomyia sand flies supported by recent experimental studies and field surveys in Thailand suggest the involvement of biting midges (Culicoides spp., Ceratopogonidae) in transmission (7–9).
Approximately a decade after cases of L. martiniquenis infection were reported in Germany and Switzerland, we present 4 independent cases of cutaneous leishmaniosis in horses outside the known range of leishmaniasis in Europe. Our report includes a phylogenetic analysis of the detected isolates and results of a pilot serologic examination.
L. martiniquensis was identified in 4 sport horses during 2019–2023 (Table). Case 1 (identified in May 2019) was in a 4-year-old Akchal-Teke mare admitted to the veterinary clinic of the University of Veterinary Sciences in Brno, Czech Republic. The mare had several small nodules (3–10 mm) on the left upper eyelid; the largest was localized near the medial canthus, measuring ≈1 cm in diameter. The mare lived in north Moravia and had been imported from Ukraine 2 years previously without any obvious lesions. Equine sarcoid was suspected on the basis of clinical examination, and bovine papillomavirus type 1 was detected by PCR in the skin smear. Case 2 (identified in May 2021) was in a 5-year-old Kladruber mare from a large stud farm that was admitted to the clinic with a group of small nodules (5–15 mm) located unilaterally on the facial area near to the lower eyelid. Case 3 was in a 5-year-old Fjord mare seen in May 2021 by veterinarians at the Equine Clinic of the Veterinary University (Vienna, Austria) with nodular lesions on the lower eyelid, chest, and udder; Leishmania was detected in the eyelid and udder lesions and bovine papillomavirus was detected in all 3 lesions. Case 4 was in a 12-year-old gelding living in the northwestern Czech Republic, first seen by the veterinarian in January 2023 for lesions on the left facial area. The clinical manifestation was very similar to those seen in cases 1–3. Again, the lesions were initially suspected to be sarcoid tumors, but the surface eventually exulcerated into an open wound. With supportive treatment, the lesion resolved over a period of 15 months; follow-up at 27 months showed no recurrence of lesions.
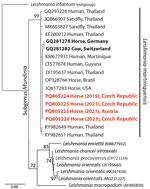
We obtained bioptic samples from cutaneous lesions using a fine needle aspiration biopsy (FNAB) for cases 1, 2, and 4 or as impression smears for case 3. We conducted routine microscopic evaluation of the FNAB material after Diff-Quick staining. Examination of the smears revealed intracytoplasmic Leishmania amastigotes in cells tentatively identified as neutrophiles (Figure 1). We cultured material obtained by FNAB from periorbital lesions (cases 1, 2, and 4) at 23°C on rabbit blood agar SNB-9 supplemented with fetal bovine serum, RPMI-1640, Schneider’s medium, and antibiotics; we then cryopreserved a single strain. Conventional PCR targeting the Leishmania internal transcribed spacer 1 (10) performed on clinical material revealed identical sequences in all 4 cases with 100% identity to other GenBank sequences of L. martiniquensis worldwide but only 99.5% concordance with previous cases in Germany and Switzerland (Figure 2).
Antibodies to Leishmania were detected by an indirect fluorescent antibody test using glass slides coated with promastigote L. infantum (VMRD, https://www.vmrd.com) and antihorse IgG (whole molecule) FITC conjugate (Sigma Aldrich, https://www.sigmaaldrich.com). We diluted serum samples in a 2-fold series starting with a 1:50 base dilution and used positive and negative control serum samples. We considered a titer >50 positive. We found antibodies to Leishmania at titers of 50 (cases 1, 2, and 3) and 100 (case 4).
We report 4 equine cases of L. martiniquensis infection outside the known range of the typical leishmaniasis caused by L. infantum in Europe, detected >12 years after the last published L. martiniquensis cases in Germany (2) and Switzerland (3). The cases occurred over a period of >3 years with no proven link between them and were also geographically dispersed across central Europe, suggesting that horses play a nonnegligible role as reservoir hosts throughout the range of L. martiniquensis. The symptomatology of L. martiniquensis cases in horses is strikingly uniform. In all 4 newly described cases, infection was diagnosed as cutaneous lesions near the eyes or in the facial area, resembling previous instances in which 7 of 10 cases were reported as lesions on the head (2,11,12).
All 4 cases were initially suspected to be sarcoid, a common skin tumor in horses caused by bovine papillomaviruses types 1, 2, and 13. Of note, in 2 cases (case 1 and 3) bovine papillomavirus types 1 and 2 were detected by PCR in lesions with Leishmania but also in lesions without the parasite. This association between sarcoid-like lesions and Leishmania infection is very suggestive. We therefore believe that the sarcoid may be attractive to blood-sucking insects (including biting midges, the potential vectors of Mundinia), thus opening the window for parasite infection. Additional cases of L. martiniquensis infection could possibly be underreported because of misdiagnosis and treatment as sarcoid or masked by a true sarcoid. Also, cases of cutaneous leishmaniosis in herbivores diagnosed in Europe should always be evaluated for the possibility of being caused by L. martiniquensis, even in areas in which L. infantum is endemic (13), particularly in the absence of sand flies.
The serologic response to L. martiniquensis remains poorly understood. A single case of cutaneous leishmaniasis in a cow revealed a robust antibody response (3). More recently, Carbonara et al. (13) reported low antibody titers in equids, including those with skin lesions or asymptomatic infections. Consistent with those observations, our findings confirm that horses with mild skin lesions exhibit only a limited antibody response. Nevertheless, serologic testing during active infection could serve as a valuable diagnostic tool.
The presence of biting midges is ubiquitous in Europe (14), and the equine population is in daily contact with them during active season. The occurrence of 4 independent cases of equine leishmaniosis caused by L. martiniquensis suggests its endemic status and circulation in Central Europe. The extent of distribution of this kinetoplastid in the equine population and other hosts in Europe remains speculative, as does its transmission biology. Given its zoonotic potential, this pathogen should be widely investigated in cases of equine skin lesions using a combination of cytology and PCR followed by sequencing. Similarly, possible L. martiniquensis infection should be anticipated in suspected visceral and cutaneous cases of human leishmaniasis, including patients without a history of travel to endemic areas.
Dr. Modrý is professor of infectious diseases of the Faculty of Science of Masaryk University in Brno and in the Department of Veterinary Sciences of the Czech University for Life Science in Prague. His interests revolve around the transmission of infectious diseases at the livestock–wildlife–humans interface, parasites as a part of biological invasions, One Health, and conservation medicine.
Acknowledgment
We thank the field veterinarians and horse owners for their assistance in sampling and information collecting.
References
- Gama A, Elias J, Ribeiro AJ, Alegria N, Schallig HD, Silva F, et al. Cutaneous leishmaniosis in a horse from northern Portugal. Vet Parasitol. 2014;200:189–92. DOIPubMedGoogle Scholar
- Müller N, Welle M, Lobsiger L, Stoffel MH, Boghenbor KK, Hilbe M, et al. Occurrence of Leishmania sp. in cutaneous lesions of horses in Central Europe. Vet Parasitol. 2009;166:346–51. DOIPubMedGoogle Scholar
- Lobsiger L, Müller N, Schweizer T, Frey CF, Wiederkehr D, Zumkehr B, et al. An autochthonous case of cutaneous bovine leishmaniasis in Switzerland. Vet Parasitol. 2010;169:408–14. DOIPubMedGoogle Scholar
- Sereno D. Leishmania (Mundinia) spp.: from description to emergence as new human and animal Leishmania pathogens. New Microbes New Infect. 2019;30:
100540 . DOIPubMedGoogle Scholar - Desbois N, Pratlong F, Quist D, Dedet JP. Leishmania (Leishmania) martiniquensis n. sp. (Kinetoplastida: Trypanosomatidae), description of the parasite responsible for cutaneous leishmaniasis in Martinique Island (French West Indies). Parasite. 2014;21:12. DOIPubMedGoogle Scholar
- Kniha E, Aspöck H, Auer H, Walochnik J. Leishmania infections and Leishmania species in central Europe. Wien Tierärztl Monat–Vet Med Austria. 2023;110.
- Bečvář T, Vojtková B, Siriyasatien P, Votýpka J, Modrý D, Jahn P, et al. Experimental transmission of Leishmania (Mundinia) parasites by biting midges (Diptera: Ceratopogonidae). PLoS Pathog. 2021;17:
e1009654 . DOIPubMedGoogle Scholar - Kaewmee S, Mano C, Phanitchakun T, Ampol R, Yasanga T, Pattanawong U, et al. Natural infection with Leishmania (Mundinia) martiniquensis supports Culicoides peregrinus (Diptera: Ceratopogonidae) as a potential vector of leishmaniasis and characterization of a Crithidia sp. isolated from the midges. Front Microbiol. 2023;14:
1235254 . DOIPubMedGoogle Scholar - Sunantaraporn S, Thepparat A, Phumee A, Sor-Suwan S, Boonserm R, Bellis G, et al. Culicoides Latreille (Diptera: Ceratopogonidae) as potential vectors for Leishmania martiniquensis and Trypanosoma sp. in northern Thailand. PLoS Negl Trop Dis. 2021;15:
e0010014 . DOIPubMedGoogle Scholar - Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HDFH, Presber W, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349–58. DOIPubMedGoogle Scholar
- Reuss SM, Dunbar MD, Calderwood Mays MB, Owen JL, Mallicote MF, Archer LL, et al. Autochthonous Leishmania siamensis in horse, Florida, USA. Emerg Infect Dis. 2012;18:1545–7. DOIPubMedGoogle Scholar
- Mendes AAV. Junior, Filgueira CPB, Miranda LFC, de Almeida AB, Cantanhêde LM, Fagundes A, et al. First report of Leishmania (Mundinia) martiniquensis in South American territory and confirmation of Leishbunyavirus infecting this parasite in a mare. Mem Inst Oswaldo Cruz. 2023;118:e220220. DOIGoogle Scholar
- Carbonara M, Mendoza-Roldan JA, Bezerra-Santos MA, de Abreu Teles PP, Lia RP, Locantore F, et al. Leishmania spp. in equids and their potential vectors in endemic areas of canine leishmaniasis. PLoS Negl Trop Dis. 2024;18:
e0012290 . DOIPubMedGoogle Scholar - Cuéllar AC, Kjær LJ, Baum A, Stockmarr A, Skovgard H, Nielsen SA, et al. Modelling the monthly abundance of Culicoides biting midges in nine European countries using Random Forests machine learning. Parasit Vectors. 2020;13:194. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: August 19, 2025
Table of Contents – Volume 31, Number 9—September 2025
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
David Modrý, Department of Veterinary Sciences, Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences Prague, Kamýcká 129, 165 21 Prague, Czech Republic
Top